Figure 3.
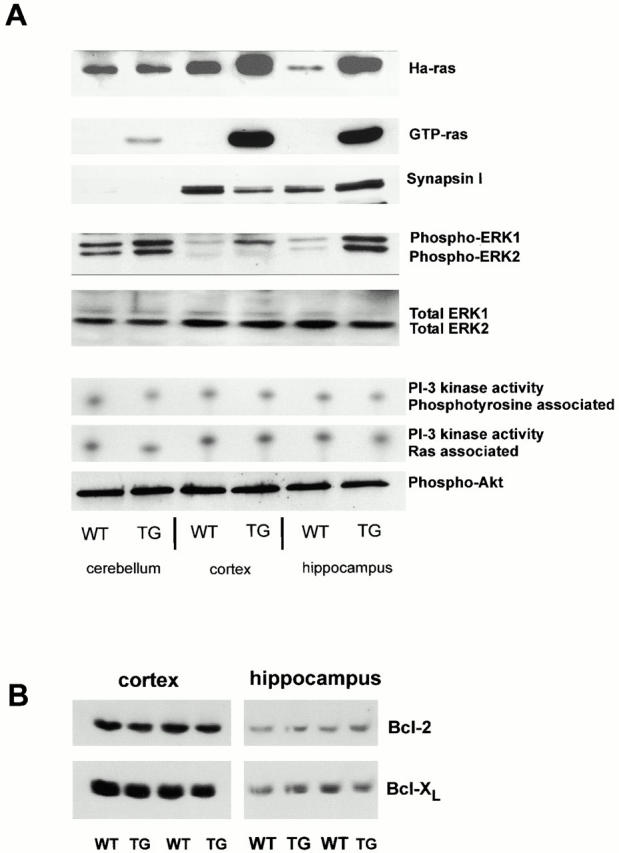
Effects of neuronal Ras-TG expression on major signal transduction pathways. (A) Ha-Ras expression: crude brain lysates were separated by SDS-PAGE and blotted on nitrocellulose. Ha-Ras expression was detected with a Ha-Ras–specific antibody. Note the increased levels of Ha-Ras protein in cortical and hippocampal brain lysates (synRas-TG). No overexpression of Ha-Ras was found in the cerebellum of synRas-TG mice. GTP-Ras levels: GTP-Ras was precipitated from crude lysates using glutathione–Sepharose loaded with a fusion protein consisting of glutathione-S-transferase and the Ras-binding domain of Raf. The affinity precipitates were separated by SDS-PAGE and blotted on nitrocellulose. The amount of signaling active GTP-bound conformation of Ras proteins was detected using a pan-Ras antibody. Transgenic V12-Ha-Ras expression results in a major increase of GTP-Ras in the cortex and hippocampus, but only an attenuated increase is observed in the cerebellum. Endogenous synapsin I expression: in adult cerebellum, the relative levels of Synapsin expression are low compared with cortex and hippocampus. Note that the Ras activity in synRas-TG mice does not regulate the endogenous Synapsin levels. MAPK phosphorylation: crude brain lysates were separated by SDS-PAGE, blotted on nitrocellulose, and MAPK phosphorylation was detected using an antibody specific for MAPK (ERK1 and ERK2) phosphorylated on the activation-competent threonine and tyrosine. In synRas-TG mice, MAPK tyrosine phosphorylation in neurons is permanently enhanced, though protein levels (ERK1 and ERK2) are not affected (see below). MAPK phosphorylation is enhanced most prominently in the hippocampus, but only moderately in the cerebellum, thus, corresponding to the different synRas-TG expression levels. In the cortex, we observe a more selective ERK1 over ERK2 phosphorylation. MAPK—total protein content: equal amounts of protein were loaded in each slot, resulting in similar signals for total amounts of ERK1 or ERK2. PI 3-kinase activity: the levels of phosphatidylinositol 3-phosphate (PI[3]P) reaction products were determined in coimmunoprecipitates using either anti-pTyr antibodies or anti-Ras monoclonal antibody 13-259. Neither the phosphotyrosine-associated, nor the Ras-associated, PI 3-kinase activity was altered. Akt (PKB) phosphorylation: crude brain lysates were separated by SDS-PAGE, blotted on nitrocellulose, and phosphorylation of the PI 3-kinase target Akt was detected using an antibody specific for Akt phosphorylated on serine 473. The phosphorylation of Akt is not altered by the Ras-TG activity in neurons. (B) Levels of Bcl-2 and Bcl-XL are not altered by the Ras-TG activity in Western blots, as determined by antibodies to Bcl-2 or Bcl-XL.
