Figure 7.
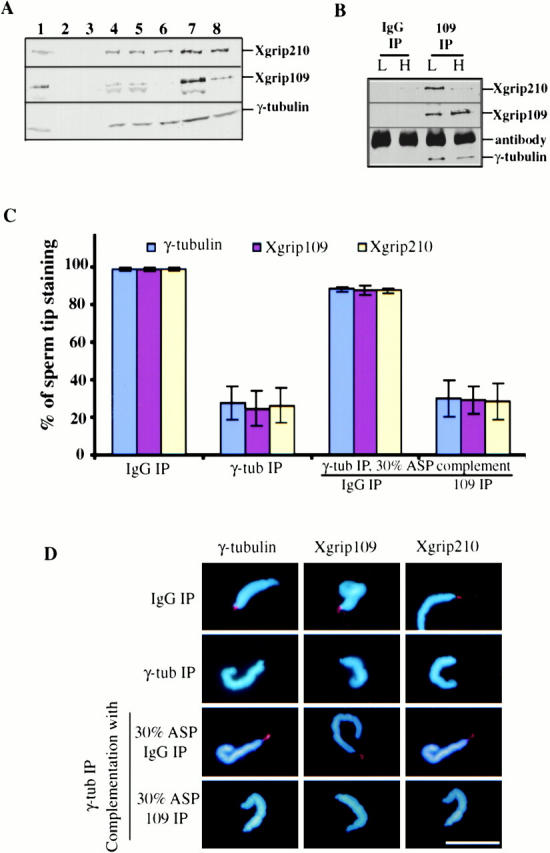
Immunodepletion of Xgrip109 inhibits the recruitment of Xgrip210 to the sperm centrioles. (A) Western blot analysis of the extracts and fractions used in the assays. Xenopus egg extract immunodepleted with either random IgG (1) or antibodies against Xenopus γ-tubulin (2). 30% ammonium sulfate supernatant (3) and pellet (4). 30% ASP resuspended in Hepes 1M and immunodepleted with either random IgG (5) or antibodies against Xgrip109 (6). 7 and 8 are the same as 5 and 6, respectively, except that the proteins were desalted into Hepes 100 and concentrated ∼10-fold. (B) Immunoprecipitates of random IgG (IgG IP) and Xgrip109 antibody (109 IP) from high (H) or low (L) salt-treated 30% ASP were analyzed by Western blot, probing with antibodies against Xgrips210, 109, or γ-tubulin. Xgrip109 antibody did not coimmunoprecipitate a significant amount of Xgrip210 in the high salt conditions. (C) Blue, red, and yellow columns represent the percentage of sperm that have γ-tubulin, Xgrip109, and Xgrip210 staining at the tips, respectively. Immunodepletion of γ-tubulin from Xenopus egg extracts (γ-tub IP) resulted in >70% reduction of the recruitment of γ-tubulin, Xgrip109, or Xgrip210 to the sperm tips, while the same treatment with random IgG (IgG IP) had no effect. Complementing the γ-tubulin–depleted egg extract with the 30% ASP immunodepleted with random IgG (IgG IP) restored the localization of γ-tubulin, Xgrip109, and Xgrip210 to the sperm tips, while the same treatment with anti–Xgrip109 antibodies (109 IP) had no effect. Error bars: standard deviations calculated from three independent experiments. (D) Representative sperm from different treatments stained with antibodies against γ-tubulin, Xgrip109, or Xgrip210 are shown. In cases where the images appear to be similar, it is due to double labeling of γ-tubulin and Xgrip210. Scale bar: 20 μm.
