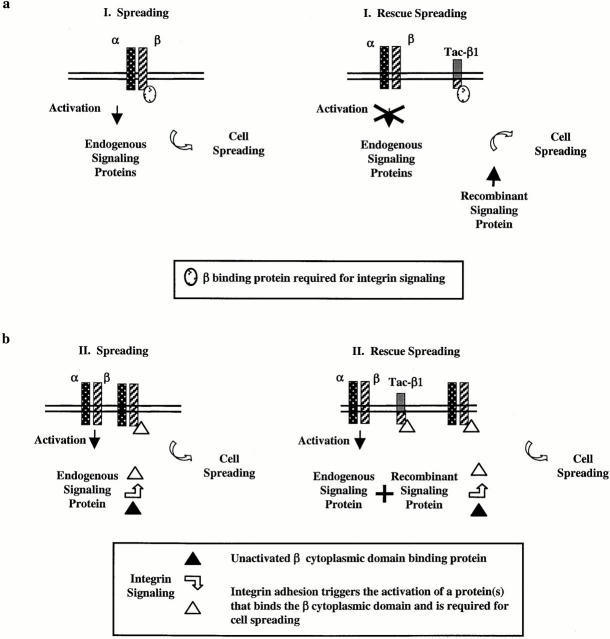
Figure 9.
Models for the rescue of tac-β1–inhibited cell spreading. (a, Model I) Expression of tac-β1 results in the titration of cellular factors from the endogenous β cytoplasmic domains that trigger the activation of signaling proteins required for cell spreading. The coexpression of activated recombinant signaling proteins with tac-β1 may bypass this block in integrin signaling and restore cell spreading. Tac-β1 does not inhibit integrin β cytoplasmic domain function that is required downstream of these signaling proteins. (b, Model II) Integrin-mediated cell attachment activates signaling proteins that regulate the binding of proteins to endogenous β cytoplasmic domains that are required for cell spreading. Expression of tac-β1 titrates these adhesion-induced protein interactions with the integrin β cytoplasmic domains. Coexpression of activated signaling proteins with tac-β1 increases the pool of proteins that bind the integrin β cytoplasmic domain and restores endogenous integrin function in cell spreading. (Model III). Tac-β1 inhibits the adhesion-induced activation of these signaling proteins and additionally inhibits protein interactions with the β cytoplasmic domain that are triggered by the signaling proteins (not shown).