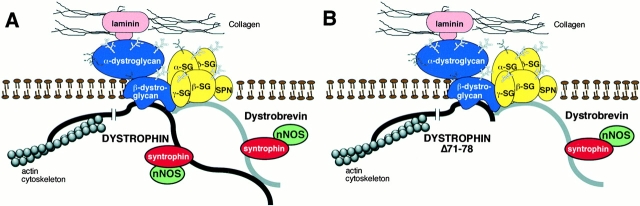
Figure 7.
Schematic diagrams of the dystrophin-associated protein complex. (A) Structure of the DAP complex in normal mouse skeletal muscle. This model reflects the presence of an interaction between dystrobrevin and the sarcoglycans and/or β-dystroglycan, in addition to dystrophin, as discussed in the text. Note that alternate binding sites to as yet unidentified DAP components are possible. (B) Proposed structure of the DAP complex in dystrophin Δ71–78 transgenic mdx mice. An interaction between dystrobrevin and the integral membrane components of the DAP complex is sufficient to localize syntrophin and dystrobrevin to the membrane in the absence of the dystrophin COOH-terminal domain.