Figure 4.
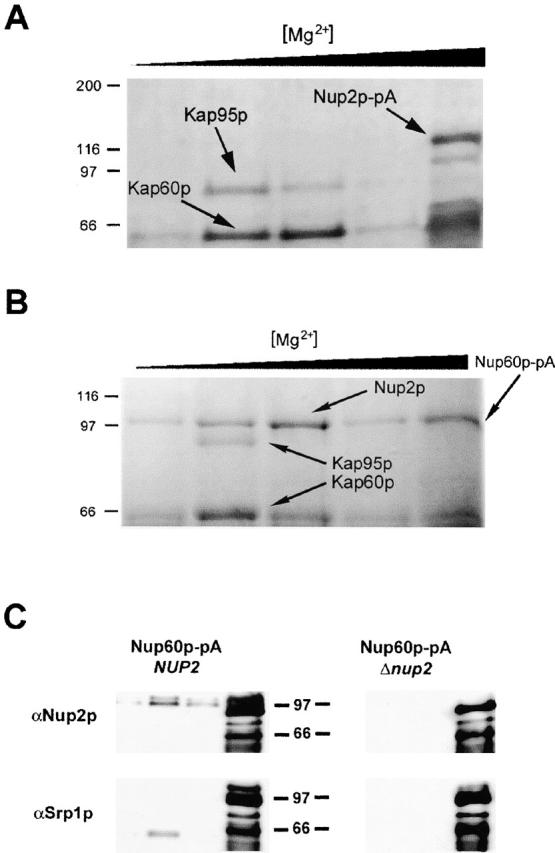
Nup2p docks at the NPC through an interaction with Nup60p and facilitates the formation of a tetrameric complex between Nup60p, Kap60p, Kap95p, and Nup2p. (A) Nup2p–pA from whole cell lysates was bound to IgG–Sepharose. Coprecipitating proteins were eluted with a MgCl2 gradient, separated by SDS-PAGE, and detected by Coomassie blue staining. Shown, from left to right, are molecular mass standards (kD) followed by the fractions eluted by treatment with 0.2, 0.5, 1.0, 2.0, and 4.0 M MgCl2. The two abundantly copurifying proteins were identified by mass spectrometry as Kap60p and Kap95p. No copurifying nucleoporins were detected. (B) Nup60p was immunoprecipitated from yeast whole cell lysates and analyzed as in A. Immunoprecipitation of Nup60p–pA from whole cell lysates coprecipitated Kap60p, Kap95p, and Nup2p, suggesting that Nup60p is the nucleoporin that anchors Nup2p to the nuclear face of the NPC. We detected no coprecipitating proteins by Coomassie staining when the same immunoprecipitation was performed from a strain lacking Nup2p (data not shown). (C) Immunoblot analysis of Nup60p–pA immunoprecipitations in wild-type and Δnup2 strains confirms the absence of both Nup2p and Kap60p in the Δnup2 strain. The 0.5, 1.0, 2.0, and 4.0 M MgCl2 elution fractions from Nup60–pA immunoprecipitations in wild-type and Δnup2 strains were probed using anti-Kap60p (anti-SRP1) and anti-Nup2p antibodies. The absence of Kap60p in the immunoprecipitation from strains lacking Nup2p indicates that Nup2p facilitates the interaction between Nup60p and Kap60p and suggests that the interaction between Nup2p and Nup60p is direct. The two closely migrating bands recognized by the anti-Nup2p antibody are specific to Nup2p as neither band is present in strains lacking Nup2p. The signal observed in the 4,000 mM elution fraction represents Nup60–pA and Nup60p–pA breakdown products that bound to the rabbit polyclonal antibodies.
