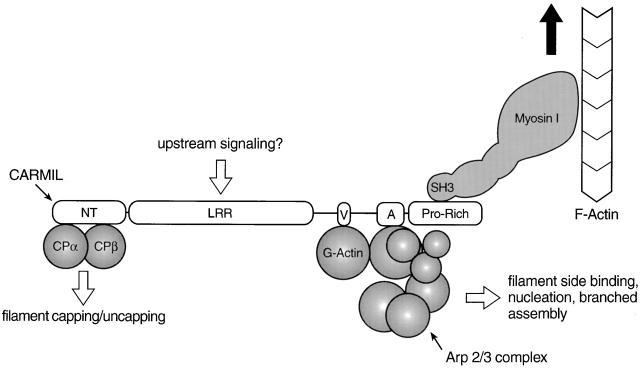
Figure 12.
Working model for the complex between myosin I, CARMIL, Arp2/3, and capping protein. In this working model, CARMIL serves as the scaffold for assembly of the complex. CARMIL, and the most conspicuous domains within it (NT, NH2-terminal domain; LRR, leucine-rich repeat domain; V, verprolin-like sequence; A, acidic domain; Pro-Rich, proline-rich COOH-terminal domain), are shown approximately to scale, whereas the remaining proteins are not. The binding of G-actin is inferred from the sequence of CARMIL and the activation data. The large black arrow signifies that myosin I might move the complex towards the barbed end of actin filaments, making this complex the “cargo”. The model also suggests that the LRR domain may be the site where upstream signaling molecules bind (e.g., Ras-related GTPases), based on the fact that the LRR domains in yeast adenylate cyclase (Suzuki et al. 1990) and the mouse protein rsp-1 (Masuelli and Cutler 1996) bind ras-related GTPases. If this were also the case for the LRR domains of p116 and Acan 125, it would provide a mechanism through which the activities of the complex could be regulated by signal transduction pathways known to regulate actin assembly in other contexts. The fact that we obtained complexes from the SH3 column in which both capping protein and the Arp2/3 complex appear to be approximately stoichiometric with CARMIL makes it very unlikely that their interaction with CARMIL is mutually exclusive. With regard to dependent binding, the fact that we sometimes obtained complexes with much more capping protein than Arp2/3 argues that capping protein does not bind to CARMIL through Arp2/3. Having said this, we never obtained complexes in which the amount of Arp2/3 complex exceeded that of capping protein. Based on this observation alone, we cannot exclude the possibility that Arp2/3 binds to CARMIL through capping protein. However, this seems unlikely based on our demonstration that different parts of CARMIL are responsible for binding capping protein and for activating Arp2/3, and based on the fact that no one has reported that capping protein binds the pseudobarbed end of the Arp2/3 complex.