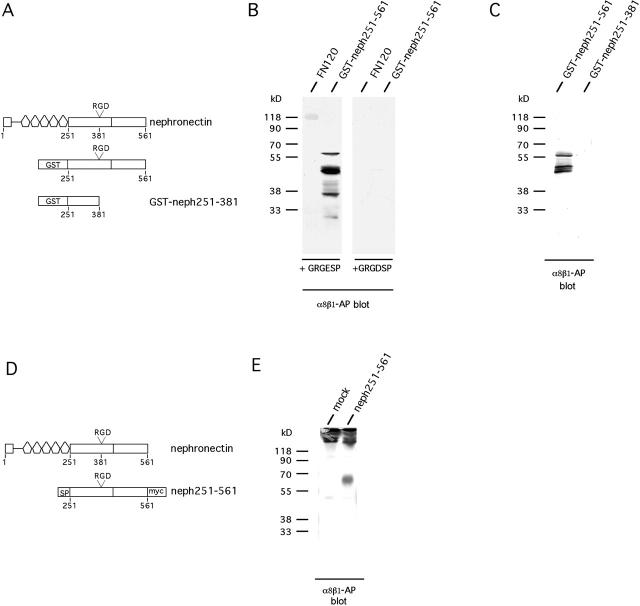
Figure 3.
The RGD-containing region in nephronectin is required for binding by α8β1. (A) Schematic representation of E. coli fusion proteins. GST-neph251-561 contains the COOH-terminal part of nephronectin from immediately after the EGF repeats to the end of the protein, whereas GST-neph251-381 contains the fragment from the end of the EGF repeats to just in front of the RGD motif. α8β1-AP binds in an RGD-inhibitable manner to nephronectin. 2 μg of FN, GST-neph251-561, or GST-neph251-381 were separated on a 12% SDS-PAGE, transferred to nitrocellulose, and probed with α8β1-AP in the presence of 2 mM Mg2+. (B) GRGDSP or GRGESP peptides were added at 50 μg/ml. Note that, under the conditions used, binding of α8β1-AP to FN is weaker than to GST-neph251-561. This blot was developed longer than the blot in C to show the FN binding. Therefore, more degradation products of GST-neph251-561 are seen than in C. (C) The RGD region of nephronectin is required for binding of α8β1-AP to nephronectin. A GST fusion protein lacking the RGD-containing region and more COOH-terminal sequences does not bind the α8β1-AP. (D) Schematic representation of fusion protein expressed in COS7 cells. Neph251-561 contains an IgG signal peptide, a fragment of nephronectin extending from the COOH terminus of the EGF repeats to the end of the protein with a myc/His tag at the COOH terminus. SP, Ig κ signal peptide; myc, myc/his tag. (E) Nephronectin fragment expressed in eukaryotic cells binds to α8β1-AP. Neph251-561 protein was transiently expressed in COS-7 cells. Then, the supernatant was harvested and immunoprecipitated with anti-myc antibody. Immunoprecipitates were separated by 12% nonreducing SDS-PAGE, transferred to nitrocellulose, and probed with α8β1-AP. mock, empty plasmid negative control. The strong band at the top of the gel represents nonreduced 9E10 Ig.