Figure 8.
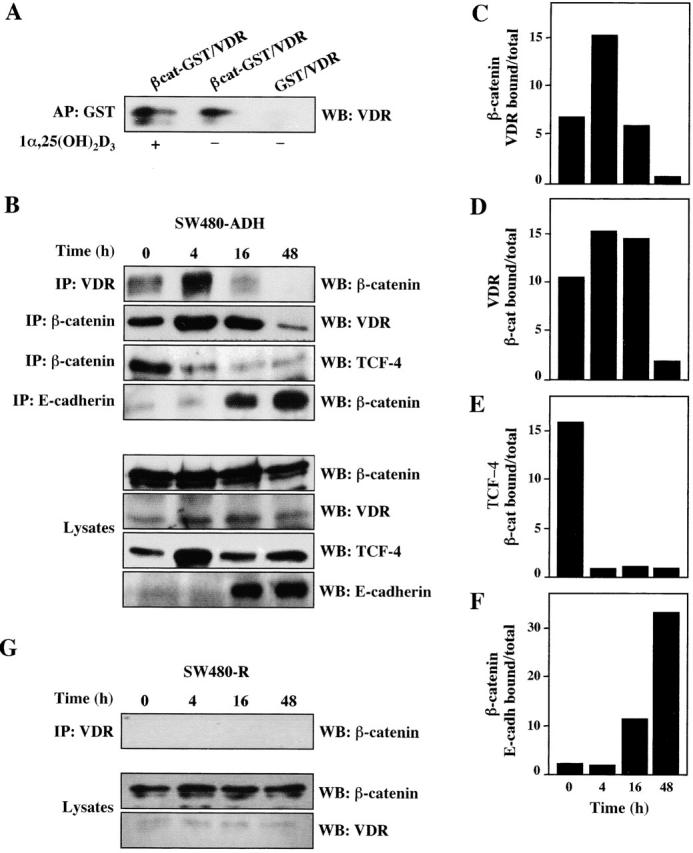
Induction of VDR–β-catenin interaction by 1α,25(OH)2D3. (A) Interaction in vitro. In vitro–translated human VDR and bacterially produced GST– β-catenin were incubated in the presence or absence of 1α,25(OH)2D3 as described in Materials and methods. Western blot (WB) analysis using a specific anti-VDR antibody of the material precipitated upon incubation with GSH–Sepharose beads. GST alone was used in parallel to rule out unspecific binding. (B) Interaction in vivo. Extracts of SW480-ADH cells untreated or treated with 1α,25(OH)2D3 (10−7 M) for the indicated times were subjected to immunoprecipitation (IP) with anti-VDR, or β-catenin, or anti-E-cadherin antibodies followed by Western blot with the antibodies indicated in each case. Western blot analysis showing the proportion of each protein present in the lysates. (C) Quantification of the amount of β-catenin bound to VDR after 1α,25(OH)2D3 addition. (D) Quantification of the amount of VDR bound to β-catenin after 1α,25(OH)2D3 addition. (E) Quantification of the amount of TCF-4 bound to β-catenin after 1α,25(OH)2D3 addition. (F) Quantification of the amount of β-catenin bound to E-cadherin after 1α,25(OH)2D3 addition. (G) Absence of VDR–β-catenin coimmunoprecipita- tion in SW480-R cells. Extracts of SW480-R cells untreated or treated with 1α,25(OH)2D3 (10−7 M) for the indicated times were subjected to immunoprecipitation with anti-VDR antibody followed by Western blotting using anti–β-catenin antibody. Western blot analysis of the lysates show the presence of only residual level of VDR.
