Abstract
Immature dendritic cells (DCs) sample their environment for antigens and after stimulation present peptide associated with major histocompatibility complex class II (MHC II) to naive T cells. We have studied the intracellular trafficking of MHC II in cultured DCs. In immature cells, the majority of MHC II was stored intracellularly at the internal vesicles of multivesicular bodies (MVBs). In contrast, DM, an accessory molecule required for peptide loading, was located predominantly at the limiting membrane of MVBs. After stimulation, the internal vesicles carrying MHC II were transferred to the limiting membrane of the MVB, bringing MHC II and DM to the same membrane domain. Concomitantly, the MVBs transformed into long tubular organelles that extended into the periphery of the cells. Vesicles that were formed at the tips of these tubules nonselectively incorporated MHC II and DM and presumably mediated transport to the plasma membrane. We propose that in maturing DCs, the reorganization of MVBs is fundamental for the timing of MHC II antigen loading and transport to the plasma membrane.
Keywords: antigen presentation; dendritic cells; endosomes; lysosomes; major histocompatibility complex
Introduction
Dendritic cells (DCs)* play a crucial role in the immune system, since they uniquely initiate primary T cell responses (Hart, 1997; Banchereau and Steinman, 1998). Antigen loading of major histocompatibility complex class II (MHC II) molecules in DCs is a tightly regulated process associated with maturation of these cells and induced by proinflammatory stimuli (Mellman et al., 1998). Immature DCs reside in peripheral tissues where they sample their environment for antigens (for example, pathogens; Sallusto et al., 1995) by receptor-mediated endocytosis, macropinocytosis, and phagocytosis (for review see Regnault et al., 1999). Upon receiving a maturation stimulus, DCs are transformed into potent antigen-presenting cells capable of activating naive T lymphocytes. In addition to the coordinate expression of chemokines and chemokine receptors (Dieu et al., 1998; Tang and Cyster, 1999) and their migration to the draining lymph organs, DC maturation is characterized by increased surface expression of MHC I, MHC II, and multiple costimulatory molecules (for example, CD40, CD80, and CD86) (for review see Hart, 1997). An increase in surface expression of MHC II is accomplished mainly by the redistribution of a presynthesized pool from intracellular storage sites to the plasma membrane (Sallusto et al., 1995; Pierre et al., 1997b; Winzler et al., 1997). The intracellular storage sites of MHC II in immature DCs are similar to those in other MHC II–expressing cells and have the characteristics of late endocytic multivesicular bodies (MVBs) or lysosomes. Collectively, these compartments are referred to as MHC II–enriched compartments (MIICs) (Peters et al., 1991; Kleijmeer et al., 1997; Geuze, 1998). In MIICs, MHC II molecules are loaded potentially with peptides that are derived from endocytosed proteins for presentation to helper T lymphocytes (for review see Watts, 1997; Geuze, 1998; Mellman et al., 1998).
Transport of newly synthesized MHC II to the endocytic tract requires its association with invariant chain (Ii) (for review see Cresswell, 1996). In MIICs, Ii is processed proteolytically to a polypeptide, the CLIP fragment, which remains associated with the peptide-binding groove of MHC II (Blum and Cresswell, 1988; Roche and Cresswell, 1991; Germain, 1994). Upon DC stimulation, the CLIP fragment can be replaced by antigen-derived peptide present in the MIIC with the aid of the accessory molecules HLA-DM (H2-M or H2-DM in mice, further called DM) and HLA-DO (H2-O; Kropshofer et al., 1999; for review see Alfonso and Karlsson, 2000). Although the regulation of MHC II peptide loading in DCs has been studied extensively (Pierre and Mellman, 1998; Inaba et al., 2000; Pierre et al., 2000; Turley et al., 2000), many questions remain. For example, the mechanism of retention of MHC II by MIICs in immature DCs and the process by which MHC II is transferred from their peptide-loading site to the plasma membrane have not been solved (Pond and Watts, 1999; Ramm et al., 2000; Turley et al., 2000).
The most prominent type of MIIC in immature DCs is the MVB (Nijman et al., 1995; Thery et al., 1999). MVBs are present ubiquitously in all eukaryotic cells. They are 300–500 nm vacuoles with numerous 50–80 nm membrane vesicles in their lumen. Although MVBs have been described as such for the first time in 1959 (Sotelo and Porter, 1959), others had reported already similar structures in 1955 (Bernard et al., 1955; Palade, 1955; Palay and Palade, 1955; Yamada, 1955). To date, the functionality of this remarkable architecture has remained elusive. The internal vesicles are formed by invaginations of the limiting membrane budding into the lumen of the MVB (Hirsch et al., 1968; Pan et al., 1985) by a process that requires PI-3 and PI-5 kinase activities (Odorizzi et al., 1998; Fernandez-Borja et al., 1999). MVBs contain many acid hydrolases, degrading a wide variety of protein and lipid substrates. Thus, one fate of the internal vesicles is the degradation of their components. A well-characterized example is the epidermal growth factor receptor, which is endocytosed and accumulates at the MVB's internal membrane vesicles only after binding of a cognate ligand (Felder et al., 1990; Renfrew and Hubbard, 1991). A second defined role for MVBs stems from their ability to fuse with the plasma membrane. When this occurs, the internal vesicles are released into the extracellular milieu (Johnstone et al., 1987; Raposo et al., 1996). These secreted vesicles have been termed exosomes, and little is known regarding their role in vivo (for review see Denzer et al., 2000). Interestingly, fusion of the MVB with the plasma membrane has also been proposed as a mechanism to transfer MHC II/peptide complexes to the cell surface. However, this process would only transfer MHC II from the MVB limiting membrane (Raposo et al., 1996; Thery et al., 1999). We find that the majority of the MHC II in MVBs is present on the internal vesicles, indicating that this fusion event would result in inefficient delivery of MHC II to the plasma membrane. The observation that secretion of MHC II carrying exosomes is decreased during DC maturation (Thery et al., 1999) also argues that other transport mechanisms are involved in the rapid and efficient transfer of MHC II to the cell surface of DCs. One such mechanism may involve transport vesicles budding from MIICs. Although clathrin coats have been identified on MIICs in melanocytes, they do not recruit selectively peptide-loaded MHC II molecules (Ramm et al., 2000). As an alternative, an intermediate compartment termed class II vesicles (CIIVs) could be responsible for the transfer of MHC II from the MVB to the cell surface (Turley et al., 2000).
In the present study, we show that in immature DCs the internal vesicles of MVBs function as a storage site for MHC II. When DCs were stimulated to mature, the MHC II carrying internal vesicles were transferred to the MVB limiting membrane. Importantly, this transfer of MHC II from the internal vesicles to the DM-containing limiting membrane possibly facilitated MHC II antigen loading. The reorganization of membrane also resulted in a striking tubular outgrowth of MIICs. The tubular compartments were directed towards the cell surface and formed MHC II carrying vesicles at their tips. These vesicles presumably transported MHC II to the plasma membrane. Collectively, our data indicate that structural changes of MVBs in DCs are crucial for the regulation of antigen presentation.
Results
MHC II expression at the cell surface is upregulated during DC maturation
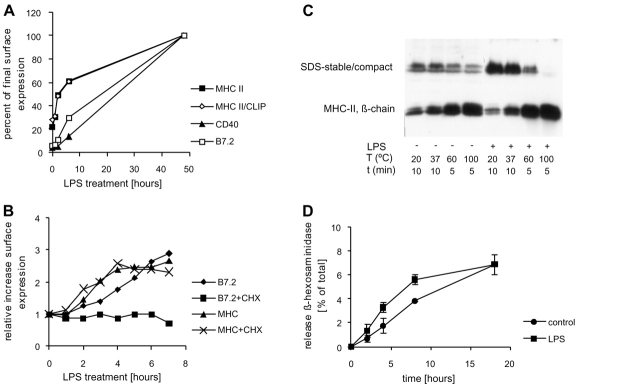
To study transport of MHC II molecules from their intracellular storage site, the late endosomal/lysosomal MIICs, to the cell surface we used D1 cells. D1 is a well-characterized growth factor–dependent long term culture of isolated splenic mouse DCs that behaves identical to freshly isolated DCs (Sallusto et al., 1995; Pierre et al., 1997a; Winzler et al., 1997). Stimulation of D1 cells with living bacteria or bacterial compounds like lipopolysaccharide (LPS) results in increased surface expression of MHC II and costimulatory molecules (Rescigno et al., 1997, 1998; Winzler et al., 1997) and a concomitant depletion of the intracellular pool of MHC II molecules from MIICs (Rescigno et al., 1997). The kinetics with which MHC II and costimulatory molecules were expressed at the plasma membrane were determined by FACS® analysis (Fig. 1 A). After a lag of ∼1 h, the surface expression of MHC II rapidly increased after LPS treatment, and half maximal effects were obtained between 2 and 6 h, in line with the notion that MHC II is recruited from a preexisting intracellular pool. 2 d after addition of LPS, the amount of MHC II at the plasma membrane was increased fivefold compared with immature cells. In contrast to MHC II, surface expression of the costimulatory molecule CD40 increased linearly with a lag of at least 3 h after LPS stimulation, consistent with transcriptional regulation. The expression of the costimulatory molecules CD80 (B7.1, unpublished data) and CD86 (B7.2) also increased with kinetics intermediate to those of MHC II and CD40. To analyze the contribution of de novo synthesis on the increase in cell surface expression of MHC II and B7.2, cells were stimulated with LPS in the presence of cycloheximide, a drug that blocks protein synthesis. The increase in cell surface expression of MHC II in response to LPS was not affected by cycloheximide, whereas the upregulation of B7.2 on the surface was inhibited strongly (Fig. 1 B). We conclude that the increased surface expression of MHC II but not that of B7.2 was due to its redistribution from an intracellular storage site. Surprisingly, CLIP-associated MHC II appeared at the plasma membrane with kinetics indistinguishable from total MHC II, indicating that peptide loading was not required for MHC II transport to the surface (Fig. 1 A). Finally, we determined the stability of MHC II heterodimers in SDS as a measure for peptide loading (Fig. 1 C). In contrast to intact Ii or CLIP-associated MHC II, peptide-loaded MHC II molecules are stable usually in the presence of SDS at room temperature and can be detected as a complex of ± 60 kD by Western blot analysis (Davidson et al., 1991; Germain and Hendrix, 1991). When immature D1 cells were lysed at room temperature in SDS sample buffer, ∼50% of the MHC II β-chain remained in a complex with the MHC II α-chain. When the temperature was elevated to 37, 60, or 100°C, increasing amounts of MHC II became unstable, but surprisingly a significant amount of the complex remained even at 100°C. In contrast, when D1 cells were treated with LPS for 2 d before lysis the majority of the MHC II complex was stable in the presence of SDS at room temperature but completely dissociated at 100°C. These data indicate that the amount of MHC II that was loaded with peptide increased significantly during D1 maturation. In addition, the heat stable complexes of MHC II present in immature cells were lost upon maturation, possibly due to degradation or additional peptide editing by DM (for review see Jensen et al., 1999).
Figure 1.
LPS-induced expression of MHC II and costimulatory molecules at the plasma membrane. (A) D1 cells were cultured in 96-well plates and incubated for 0, 1, 3, 6, or 48 h with LPS. The surface expression of MHC II, CLIP-associated MHC II (MHC II/CLIP), CD40, and B7.2 was analyzed by FACS®. (B) Cells were cultured as above for 0, 1, 2, 3, 4, 5, 6, and 7 h with LPS either in the presence or absence of cycloheximide. Surface expression of B7.2 and MHC II was analyzed by FACS®. (C) Cells were lysed after 48 h of culture in the presence or absence of LPS in SDS sample buffer. Samples were incubated at room temperature, 37, 60, or 100°C and analyzed by Western blotting for MHC II β-chain. The Western blot shows monomeric MHC II β-chain and SDS stable MHC II. Upon stimulation with LPS, the amount of SDS stable MHC II/peptide complexes at room temperature strongly increased on expense of SDS unstable MHC II molecules. In contrast, the amount of SDS stable complexes at 100°C decreased after maturation. (D) Immature D1 cells secreted ∼7% of their β-hexosaminidase in a linear fashion during 18 h. The release increased only slightly after 4 and 8 h of LPS treatment but was unchanged at 18 h.
LPS induces tubulation of MIICs
As a result of the massive transport of MHC II molecules from MIICs to the cell surface within a short time period, we anticipated to see this transport reflected in the morphology of the endocytic compartments/MIICs upon stimulation with LPS. First, we applied whole-mount EM (Stoorvogel et al., 1996) to obtain an overall morphological three-dimensional impression of the endocytic system (Figs. 2 and 3). This technique reveals the overall shape and characteristics of endosomes and lysosomes. Immature and LPS-stimulated D1 cells were allowed to endocytose HRP as a fluid phase marker for 1 h at 37°C and were then incubated at 0°C with diaminobenzidine (DAB) in the presence of H2O2. DAB diffuses through membranes and is rapidly polymerized in HRP-containing compartments. Only endosomal and lysosomal proteins are cross-linked to DAB polymer by this procedure, allowing removal of nonlinked cytosolic proteins after subsequent permeabilization of the plasma membrane with saponin. Saponin-treated cells were then fixed with aldehydes, immunolabeled with colloidal gold, and studied as whole-mount preparations by transmission EM. The most striking difference that we observed between LPS-treated and nontreated cells was a shape change of the endocytic compartments/MIICs. Immature DCs were characterized by the presence of numerous electron-dense vacuolar MIICs, which localized predominantly to the perinuclear area (Fig. 2 A). The nucleus and cytoskeletal elements can be seen as electron-dense structures also. Fig. 2 B shows a maturing DC that was incubated for 1 h with HRP after which LPS was added for 6 h. At this time, the cells were in the process of actively redistributing MHC II to the cell surface (Fig. 1 A). Most notably, the vacuolar MIICs were transformed into tubular MIICs, which extended towards the cell periphery (Fig. 2 B, arrows). A similar morphology was obtained after a 3-h chase in the presence of LPS (unpublished data).
Figure 2.
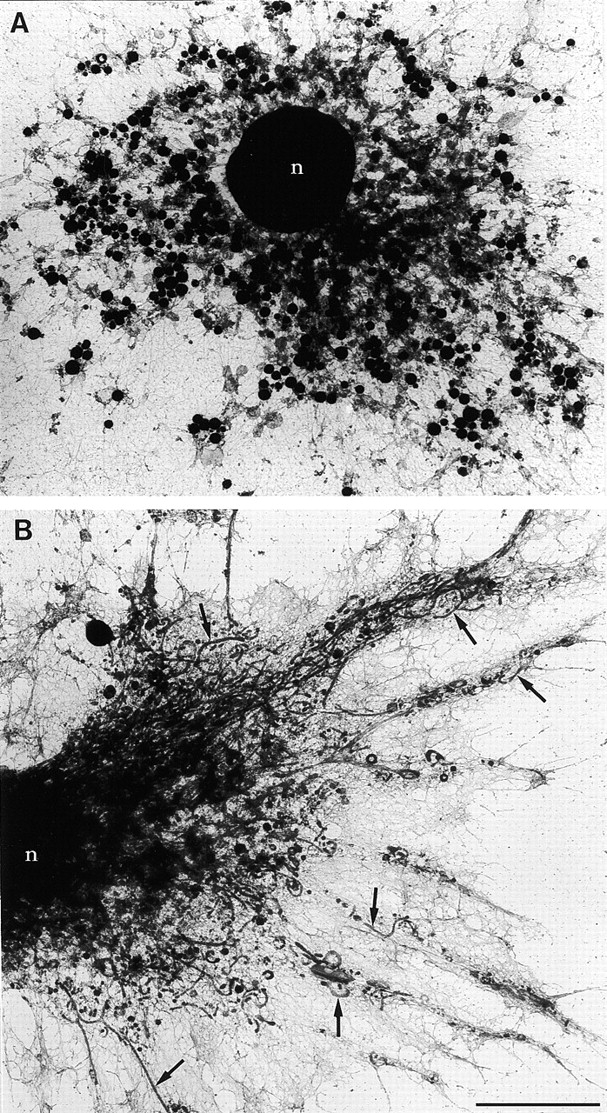
LPS-induced remodeling of the endocytic system shown by whole-mount EM. For the inspection of endosomes and lysosomes (MIICs) in overview at the EM level, D1 cells were allowed to endocytose HRP as a fluid phase marker for 1 h (A) or were loaded for 1 h with HRP after which LPS was added for 6 h (B). Cells were chased for 10 min in the absence of HRP and then processed for whole-mount viewing of HRP-containing compartments. (A) Unstimulated cell with numerous vacuolar MIICs. (B) Stimulated cell with drastically remodeled long MIIC tubules extending into the dendrites of the cell (arrows). n, nucleus. Bar, 4 μm.
Figure 3.
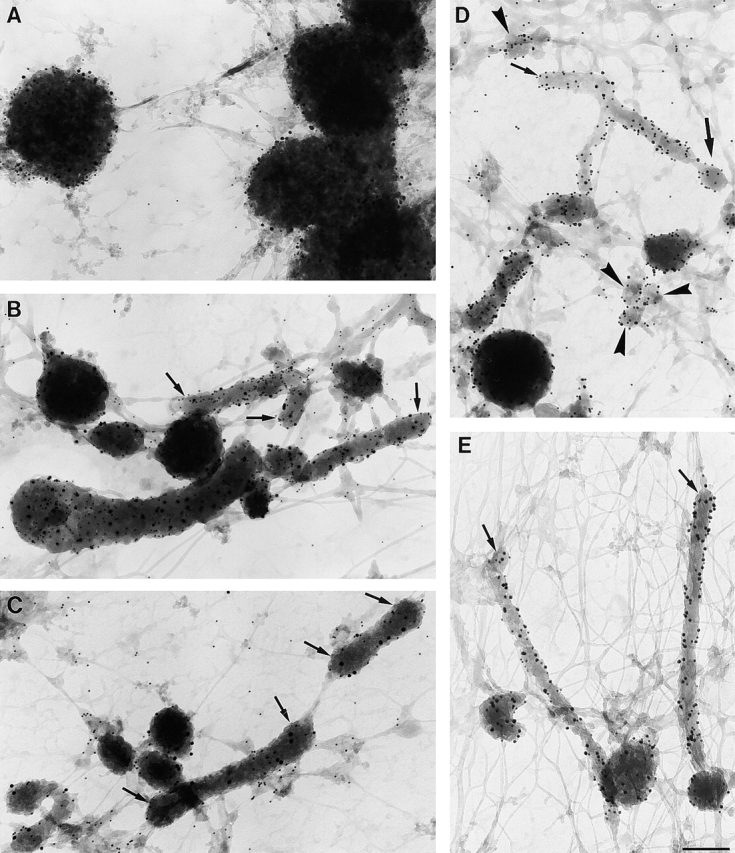
MIICs in immature and maturing D1 cells. Cells were loaded with HRP for 1 h in the absence of LPS as in the legend to Fig. 2 A (A) or 1 h in the presence of HRP plus 6 h HRP + LPS (B and C) or pulsed for 1 h with HRP and chased for 3 h in the presence of LPS (D and E) and processed for whole-mount EM. (A) Vacuolar MIICs in immature D1 cells labeled for MHC II (10 nm gold) and DM (15 nm gold). Tubular MIICs have formed after 3 (D and E) or 6 h (A and B) of LPS treatment. (B) Double labeling for MHC II (10 nm gold) and DM (15 nm gold). A tubule seems to form out of the vacuole in the left bottom corner. (C) Tubular MIICs labeled for MHC II (10 nm gold) and LAMP-1 (15 nm gold), demonstrating the late endosomal/lysosomal character of these organelles. (D) Tubular MIICs and free vesicles (arrowheads) with diameters ranging from 80 to 200 nm were double labeled for MHC II (10 nm gold) and DM (15 nm gold). Note the vesicle that seems to be formed at the tip of a tubule (thick arrow). (E) Tubular MIICs, showing DM (10 nm gold) and LAMP-1 (15 nm gold). Note the cytoskeletal elements in which the MIICs are embedded.
To characterize these compartments further, whole-mount preparations of immature and LPS-treated D1 cells were double immunolabeled using antibodies that are directed against the cytoplasmic domains of relevant membrane proteins. Both the vacuolar and tubular DAB-positive compartments labeled for MHC II, DM, LAMP-1 (Fig. 3, A–E) and Ii tail (unpublished data) thereby identifying these structures as MIICs (Peters et al., 1991, 1995; Nijman et al., 1995; Kleijmeer et al., 1997). The tubular MIICs had a diameter <200 nm (Fig. 3, B–E) and seemed to form from the vacuoles (Fig. 3 B). Vesicles with a similar diameter (Fig. 3, B–E, arrows) that were also labeled for MHC II, DM, and LAMP-1 were often encountered close to the tips of the tubules (Fig. 3 D). Since the cells were pulse-chase labeled with HRP, the presence of DAB polymer in the tubules and vesicles indicated that they were formed from vacuolar MIICs. We propose that the free vesicles transport MHC II out of the tubular MIICs, probably to the plasma membrane.
MHC II is recruited from the internal membranes of MIICs
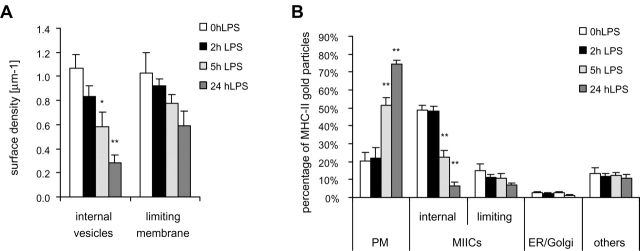
Next, we studied whether the transformation of vacuolar into tubular MIICs involved membrane rearrangements within the MIICs by performing immuno-EM on cryosections. In immature cells, MHC II was primarily associated with the 250–600 nm vacuolar multivesicular MIICs (Fig. 4, A and C), and only little was displayed at the plasma membrane (Fig. 4 A). In contrast, after 48 h of LPS, MHC II labeling was increased strongly at the plasma membrane, whereas residual MIICs were almost devoid of MHC II (Fig. 4 B). The most striking difference between MIICs in immature (Fig. 4, A and C) and mature (Fig. 4 B) cells was the disappearance of their internal membranes. After 3–6 h of LPS treatment, tubular MIICs appeared either with a dense content and only a few internal membranes or without almost any visual content (Fig. 4 D). The disappearance of internal membranes from MIICs and the coincident appearance of tubules and vesicles (diameter <200 nm) was quantified by a morphometric analysis in which the surface density of the internal membranes was determined after 0, 2, 5, or 24 h of LPS. Compared with the control, after 5 and 24 h of LPS the surface density of internal membranes decreased 2- and 4.5-fold, respectively (Fig. 5 A). Concomitantly, the surface density of MIICs limiting membrane decreased with ∼40% after 24 h. These figures are consistent with transfer of membrane from the internal vesicles to the limiting membrane of MIICs and with a net transfer of membrane from MIICs to the plasma membrane. To investigate whether the decrease in internal membranes was accompanied by a redistribution of MHC II, we determined the relative subcellular distribution of MHC II. In immature cells, intracellular MHC II was associated almost exclusively with MIICs, and only minor amounts were associated with the biosynthetic pathway (Fig. 5 B). Within the MIICs, ∼80% was associated with the internal vesicles and the remainder with the limiting membrane (Fig. 6 A). The presence of MHC II on internal membranes strongly decreased upon LPS treatment with a corresponding increase at the plasma membrane. The ER, Golgi, and undefined organelles did not contribute to the increased plasma membrane-associated pool of MHC II. Together with the notion that this increase occurred independently of de novo synthesis of MHC II (Fig. 1, A and B), these data indicate that MHC II is redistributed from the internal vesicles of MVBs to the plasma membrane.
Figure 4.
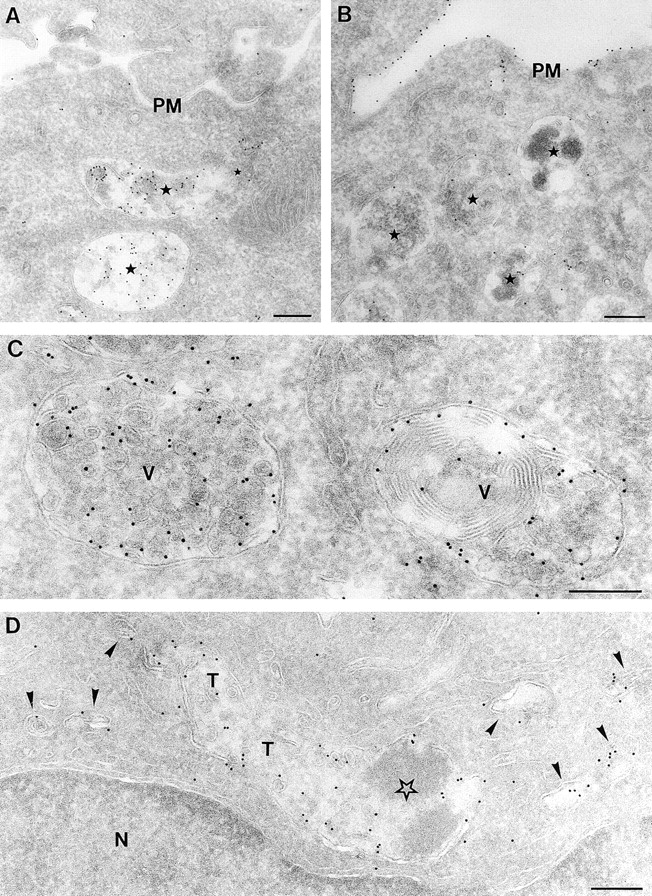
LPS-induced depletion of the MIICs internal membranes. Ultrathin cryosections of untreated D1 cells (A and C) or D1 cells treated with LPS for 48 (B) or 3 h (D) were immunolabeled for MHC II with 10 nm gold particles. In A, MHC II is primarily present in vacuolar MIICs (stars) comprised of a limiting membrane surrounding internal vesicles and membranous sheets. Only a few gold particles are present at the plasma membrane (PM). In contrast, in the LPS-treated D1 cell in B the plasma membrane is labeled strongly for MHC II, whereas only little MHC II localizes to MIICs, which have few internal membranes (stars). (C) Vacuolar MIICs (V) display many internal membrane vesicles that are labeled for MHC II, whereas the limiting membrane is labeled scarcely. (D) MHC II–positive tubules (T) and vesicles (arrowheads) after 3 h of stimulation with LPS. The dense vacuolar part is marked by a star. N, nucleus. Bars: (A and B) 250 nm; (C and D) 200 nm.
Figure 5.
Morphometric analysis of MIIC membrane domains and subcellular distribution of MHC II. Random pictures (20×) were taken of ultrathin cryosections of D1 cells incubated for 0, 2, 5, or 24 h with LPS. The sections were immunolabeled for MHC II. (A) Surface densities of internal and limiting membranes of MIICs. Significant differences were found for the surface density of internal vesicles after 5 and 24 h. (B) The change in relative distribution of MHC II gold particles over PM, MIICs, ER + Golgi complex, and others (n.d. + cytosol + mitochondria + nucleus) was determined on random pictures (*p < 0.05; **P < 0.005).
Figure 6.
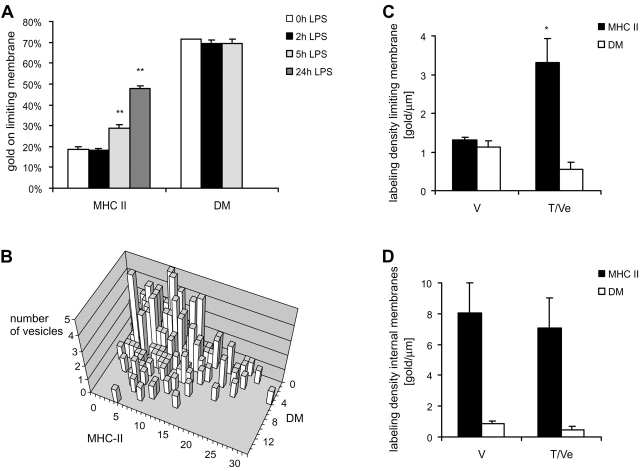
Labeling characteristics of MHC II and DM in MIICs. (A) Ultrathin cryosections of D1 cells incubated for 0, 2, 5, or 24 h with LPS were labeled for MHC II or DM. The relative amount of labeling on the limiting membrane was determined and expressed as the percentage of total labeling on MIICs. Treatment with LPS induced a clear redistribution of MHC II towards the limiting membrane, whereas the distribution of DM did not change. (B) The population of 200 DAB-containing MHC II and/or DM-labeled transport vesicles indicated in Table I were grouped according to their MHC II and DM content. Each bar represents the number of vesicles having a certain labeling characteristic. Only a single population of vesicles, containing both MHC II and DM, was observed. (C) The labeling densities of MHC II and DM on limiting and internal membranes of MIICs were measured in electron micrographs of cells treated for 3 h with LPS. Labeling densities on the limiting membranes of V and T/Ve are expressed as the ratios of gold particles over membrane surface areas (as described in Materials and methods). The labeling density of MHC II on the limiting membrane of V is less than two times that of T/Ve. (D) Labeling densities of MHC II and DM on internal membranes do not alter after LPS treatment (*p < 0.05; **P < 0.005).
LPS redistributed MHC II from the internal vesicles to the DM-containing limiting membrane of MIICs
Since MHC II together with the pool of internal membranes was depleted from MIICs, we next investigated whether these membranes could be redistributed to the limiting membrane of MVBs from where they could find their way to the cell surface. At 0 and 2 h of LPS treatment, only 20% of MHC II was located on the limiting membrane of MIICs. However, after 5 and 24 h of LPS treatment, we found 30 and 50%, respectively, of MHC II on the limiting membrane (Fig. 6 A), most likely as a result of the insertion of MHC II–enriched internal membrane. It should be noted that the extent of this redistribution is probably underestimated, since after 5 and 24 h of LPS treatment a significant amount of MHC II had been recruited already from the limiting membrane of MIICs to the plasma membrane (Fig. 1 A). When we compared the relative distribution of MHC II with that of DM, striking differences were observed. In contrast to MHC II, in immature cells DM was relatively enriched (70%) on the limiting membrane of MIICs (Fig. 6 A and Fig. 7 A). The relative distribution of DM was not effected by LPS treatment. Since DM plays an important role in MHC II peptide loading, we predicted that peptide loading of MHC II might be enhanced at the limiting membrane due to the increased concentration of DM at this domain. To test this hypothesis, we used BMDCs, long term cultured bone marrow–derived DCs from B10.A (5R) mice that express the YAe epitope. This epitope is formed by I-Ab associated with Eα (Drα)52-68 peptide (Murphy et al., 1989; Rudensky et al., 1991; Eastman et al., 1996). This peptide originates from an endogenous protein, the I-Eα chain, and loading of this peptide onto MHC II occurs in MIICs and is dependent on DM (Kovats et al., 1998).
Figure 7.
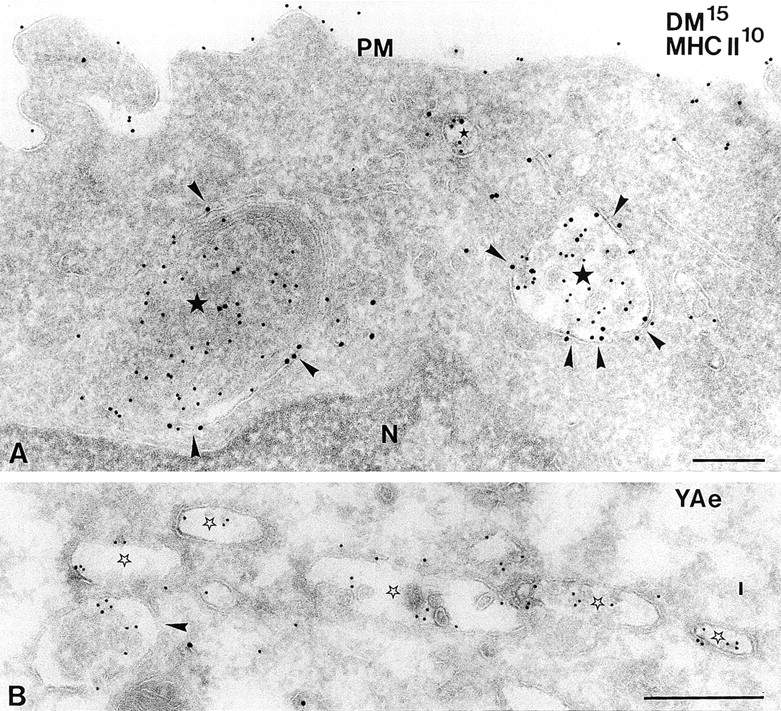
Localization of DM and YAe on the limiting membrane of MIIC. (A) Double immunolabeling of MIICs (stars) in immature D1 cells, showing DM (15 nm) primarily on the limiting membrane (arrowheads) of MIICs and most of the MHC II (10 nm) on internal membranes. N, nucleus; PM, plasma membrane. (B) Immunolabeling showing YAe on the limiting membrane of MIICs (stars) in BMDCs. Bars, 200 nm.
After 6 h of LPS treatment, MIICs in BMDCs abundantly labeled for the YAe epitope (Fig. 7 B) with 70% of the label associated with the limiting membranes. These data cannot be compared directly with those obtained for D1 cells. Nevertheless, they suggest that peptide loading of MHC II is enhanced after its redistribution to the limiting membrane of MIICs by the increased availability of DM. Therefore, the reorganization of MVBs may contribute to the regulation of MHC II peptide loading.
MHC II is not sorted from DM during its recruitment from MIICs
We next addressed the question of whether MHC II and DM were sorted during the membrane reorganization of MIICs. Incorporation of MHC II–rich/DM-poor internal membranes into the MHC II–poor/DM-rich limiting membrane was expected to result in changing densities of these molecules in the limiting membrane of MIICs. To address this point, we determined the labeling densities of MHC II and DM in the internal and limiting membranes of MIICs after 3 h of stimulation with LPS, a situation at which both vacuolar MIICs and tubules/vesicles were encountered in the same cell. On the vacuolar MIICs, the labeling density of MHC II on the limiting membrane was similar to that of DM, whereas on tubules and vesicles the density of MHC II was fivefold higher than that of DM (Fig. 6 C). It should be noted that tubules are only recognized as such when they are oriented in the plane of the section. The labeling densities of MHC II and DM at the internal membranes were the same for the two MIIC types (Fig. 6 D). At the limiting membrane, the labeling density of DM was less at the T/Ve than at the vacuoles as can be expected when this membrane is diluted with fusing internal membranes that are poor in DM. Together, these data are consistent with a model in which upon maturation internal membranes are recruited to the limiting membrane (Fig. 8).
Figure 8.
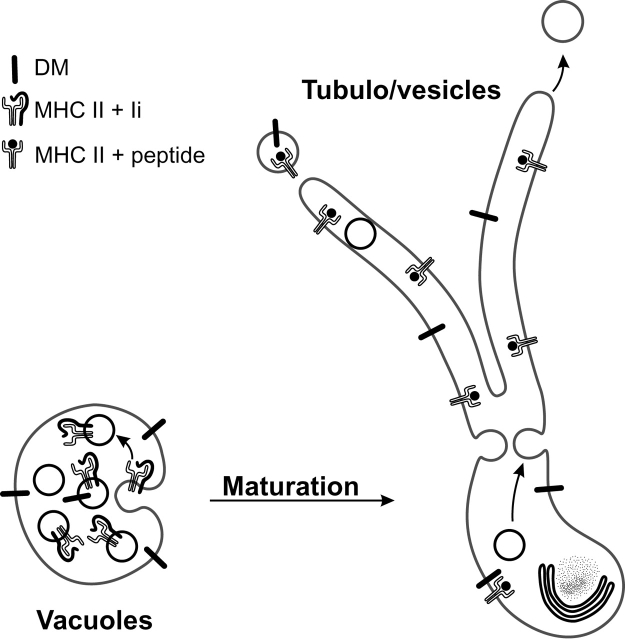
Tubulation of multivesicular MIICs during DC maturation. Multivesicular MIICs in DCs undergo a dramatic shape change from vacuolar to tubular upon stimulation, most likely by fusion of the MIIC internal membrane vesicles with the limiting membrane. This implies that MHC II–rich internal membranes stored in the lumen of the vacuolar MIIC relocate to the limiting membrane of the tubular MIICs, allowing egress of MHC II from these tubules to the plasma membrane. The final transport step to the cell surface is probably mediated by transport vesicles, which bud from the tubular MIICs and nonselectively incorporate MHC II. As a consequence of MHC II translocation to the DM-rich limiting membrane, contact between DM and MHC II is increased, which may facilitate peptide loading and editing during maturation.
To determine whether MHC II is selectively incorporated into the vesicles that derive from the tips of the tubular MIICs, we performed a quantitative analysis of the distribution of DM and MHC II on whole-mount preparations. We examined the relative labeling densities of MHC II and DM on the vesicles and on the tubular MIICs and their tips, the putative vesicle formation sites (Table I). A tip was defined as a segment at the end of each tubule with a length equal to its diameter. On the tubules, the labeling density of MHC II was 3.2-fold higher than that of DM. On buds and vesicles, similar ratios, 3.3 and 3.8, respectively, were observed, suggesting that MHC II molecules are not sorted away from DM during vesicle formation. As illustrated in Fig. 6 B, we did not observe transport vesicles that are exclusively enriched in MHC II or DM. A single population of vesicles containing both MHC II and DM was observed. This predicts that DM is transported together with MHC II from tubular MIICs to the plasma membrane by a default process. We propose that the MHC II/DM-positive vesicles and not entire tubules mediate transport to the cell surface. In the event of fusion of MIIC tubules with the plasma membrane, their entire soluble content would be quantitatively transferred to the extracellular milieu. However, when we measured the release of the lysosomal enzyme β-hexosaminidase in the medium, LPS treatment only slightly increased the secretion of the enzyme in the medium (Fig. 1 D).
Table I. DM is not segregated from MHC II during vesicle formation from MIIC tubules.
| Tubules | Buds | Vesicles | |
|---|---|---|---|
| MHC II gold particles | 2785 | 1683 | 2069 |
| DM gold particles | 885 | 513 | 545 |
| Ratio MHC II/DM | 3.2 | 3.3 | 3.8 |
Whole-mount preparations of LPS-stimulated D1 cells were double immunolabeled for MHC II and DM as in Fig. 3 D. The samples were scanned randomly for labeled tubular 100–200 nm MIICs and 100–150 nm vesicles. The tubules were divided into “buds,” defined as the distal end of a tubule with a length equal to its diameter, and the remainder of the tubule. The number of gold particles representing MHC II or DM on 200 vesicles and 94 tubules and 170 buds were counted, and the ratio of the two markers was calculated for each structure.
Discussion
In antigen-presenting cells, the main type of MIIC in which peptide loading of MHC II occurs is constituted of MVBs (for review see Geuze, 1998). Here, we present data that explain how immature DCs can efficiently retain MHC II molecules in their MIICs and recruit them from these stores to the cell surface during maturation. In immature D1 cells, >65% of MHC II was contained by their MIICs, of which 80% was associated with the internal vesicles. In contrast, DM was associated predominantly with the limiting membrane of the MIIC (70%). This differential distribution may be due to a selective retention of DM at the limiting membrane and by active incorporation of MHC II into the internal vesicles. The latter possibility seems to be the major determinant, since the density of MHC II is much higher at the internal vesicles than at the limiting membrane (Fig. 6, B–D). Already after a few hours of LPS treatment, long tubules egressed from the MVBs, reaching out into the periphery of the cell. Both the vacuolar and tubular MIICs showed the characteristics of late endosomal/lysosomal compartments as demonstrated by the presence of LAMP-1, DM, and HRP uptake kinetics. The tubulating MIICs were reminiscent of tubulo/vesicular lysosomes in macrophages that are formed after stimulation with phorbol esters (Swanson et al., 1987). In the whole-mount preparations, the tubular nature of MIICs appeared more prominent than in sections of aldehyde-fixed cells. Apart from the inherent limitation of showing tubules in sections, this was possibly also due to the fixation with aldehydes. Aldehyde fixation results in the fractionation of long tubular MIICs as reported for tubular lysosomes in macrophages (Robinson and Karnovsky, 1991). In the whole-mount procedure, MIICs were fixed by DAB polymer, and this prevented their fractionation as suggested by cryosections of HRP-loaded cells that were aldehyde fixed after DAB treatment (unpublished data). MIICs must recruit considerable amounts of additional limiting membrane to accomplish their tubulation. Our morphometric analysis indicates that this membrane most likely originates from the internal membrane vesicles of the MVBs. Fusion of internal vesicles with the limiting membrane might even drive the tubulation of these organelles. To our knowledge, this is the first demonstration that internal membrane vesicles of MVBs serve as a storage compartment from which membrane proteins can be recruited on demand.
The transport intermediates from the tubular MIICs to the plasma membrane are most likely the 80–200 nm vesicles. Several arguments favor this idea. First, their diameter is similar to that of the MIIC tubules, and they are often encountered proximate to the tips of these tubules. Second, endocytosed HRP is found in these vesicles even after chase times of 6 h, indicating that they originate from compartments that are positioned late in the endocytic tract. Third, they are, like MIICs, labeled for both MHC II and DM, demonstrating their relationship with MIICs. It is unlikely that fusion of entire MIICs with the plasma membrane provides a major pathway for MHC II traffic because the release of β-hexosaminidase was increased only slightly upon stimulation with LPS. However, fusion of entire MIICs with the plasma membrane of D1 cells does occur to some extent as illustrated by the release of exosomes (Thery et al., 1999; unpublished data). D1 cells that were stimulated for 24 or 40 h with LPS released exosomes less efficiently than immature cells (Thery et al., 1999). This observation may be explained by our finding that MIICs in the mature cells are depleted from internal vesicles. Thus, exosome secretion can be expected to go down irrespective of the frequency of MIIC fusion with the plasma membrane.
Based on our observation that in maturing D1 cells MHC II is not selectively concentrated at the tips of tubular MIICs nor on the putative transport vesicles, we conclude that sorting information is not required for MHC II to egress from MIICs. This is consistent with the observation that in a murine B lymphoma cell line, transport of MHC II to the cell surface occurs independently of the cytoplasmic domains of the α- and β-chains (Thery et al., 1998) and with the lack of recruitment of peptide-loaded MHC II molecules by clathrin coats present on MIICs (Ramm et al., 2000). That a vesicular intermediate similar to the CIIV described in B cells (Amigorena et al., 1994; Pierre et al., 1997b) is involved in transport to the cell surface has been suggested also by Turley et al. (2000) in a study on developing bone marrow–derived DCs. The CIIV is thought to move from MIICs to the plasma membrane, carrying MHC I, MHC II, and B7 costimulatory molecules. Possibly, CIIVs are similar to the putative transport vesicles or tubular structures in our study. However, one discrepancy is that CIIVs lack DM as measured by immunofluorescence, but this may be due to the higher detection efficiency of DM by whole-mount EM in our study. Since the transport vesicles in our study do contain DM, one may expect an enhanced surface expression of DM upon stimulation. Although DM was observed occasionally at the plasma membrane, we were unable to demonstrate increased DM expression upon stimulation. However, others have shown that functional DM is present at the cell surface of B cells and DCs. The reason why we did not detect increased levels of DM at the plasma membrane may be due to rapid endocytosis of surface-targeted DM (Lindstedt et al., 1995; Riese et al., 1996), resulting in regurgitation to MIICs and low surface levels at steady state. Thus, sorting between DM and MHC II in maturing DCs may occur at the plasma membrane rather than at MIICs.
During maturation, DCs upregulate MHC II peptide loading and transport to the cell surface (Pierre and Mellman, 1998; Inaba et al., 2000). In DCs from cathepsin S–deficient mice (Driessen et al., 1999) and in cells that were incubated in the presence of an inhibitor of cathepsin S (Pierre and Mellman, 1998), surface expression of MHC II was delayed severely. Since cathepsin S is required for the final processing of Ii (Riese et al., 1996; Villadangos et al., 2000), it has been proposed based on these data that the cytoplasmic domain of Ii might prevent MHC II from escaping MIICs. However, we observed that upon D1 stimulation, MHC II is recruited to the cell surface with the same kinetics as CLIP-associated MHC II, indicating that the recruitment process lacks specificity and that peptide loading is not required. Probably, Ii-associated MHC II is endocytosed rapidly upon reaching the plasma membrane and shuttled back to MIICs. Alternatively, Ii might be required to actively target newly synthesized MHC II to the internal vesicles of MIICs in immature DCs.
Our results show that DCs employ the internal membranes of their multivesicular MIICs to temporarily store large amounts of MHC II molecules and that they execute a radical reorganization of their MIICs to mediate rapid transfer MHC II to the cell surface upon activation. Maturation of D1 cells also involved increased peptide loading on MHC II and possibly editing of peptides that were associated already with MHC II before maturation (Fig. 1 C). Sorting of MHC II molecules into the internal vesicles of MVBs in immature cells by itself provides a physical restraint for egress to the cell surface. In addition, the relatively low amount of DM associated with the internal membranes may prevent peptide loading of a large pool of MHC II molecules in immature DCs. Fusion of MHC II–containing membranes with the DM-rich limiting domain of the MIIC brings the molecules together and will enhance the efficiency of peptide loading and editing by DM.
Others already concluded that peptide loading is not the only regulatory process for MHC II transport to the cell surface in maturing DCs (Pierre and Mellman, 1998; Inaba et al., 2000). In our study, cycloheximide did not interfere with the LPS-induced increase in the expression of MHC II at the plasma membrane, indicating that newly synthesized MHC II molecules did not contribute significantly to this process. This is consistent with the observation that stimulated D1 cells did not show a high increase in their synthesis of MHC II (Rescigno et al., 1998) and that the relative contribution of MHC II in the ER and Golgi complex did not change during LPS treatment (Fig. 5 A).
The regulated recruitment of internal vesicles of MVBs to their limiting membrane is an entirely new concept. For DCs, this process allows for the timed recruitment of presynthesized MHC II to the plasma membrane and possibly has a regulatory function in peptide loading. Similar mechanisms may be employed by other cells to regulate the surface expression of distinct sets of membrane proteins.
Materials and methods
Materials
D1 cells are long term cultured growth factor–dependent immature splenic DCs derived from C57BL/6 (H-2b) mice (Winzler et al., 1997). BMDCs were generated from bone marrow of B10.A (5R) mice. Cells were grown in IMDM containing 10% heat-inactivated FCS (GIBCO BRL), 100 IU/ml penicillin, 100 μm/ml streptomycin, 2 mM l-glutamine, 50 μM 2-mercaptoethanol (all from Sigma-Aldrich), and 35% conditioned medium from R1 cells (Winzler et al., 1997). BMDCs were grown for 15–20 d in DC medium, and homogeneity of the DC culture was evaluated by cytofluorimetry (unpublished data).
LPS (Escherichia coli, serotype 026:B6) was obtained from Sigma-Aldrich. Cycloheximide (Sigma-Aldrich) was used at 10 μg/ml. Rabbit polyclonal antibody directed against the cytoplasmic domains of DM (Barois et al., 1998), MHC II β-chain, and Ii (Barois et al., 1997) were obtained from Dr. Barois (University of Oslo, Oslo, Norway). Monoclonal rat anti–mouse MHC II (M5/114 and PE-coupled M5/114 [Bhattacharya et al., 1981]) and FITC-coupled CD86/B7.2 antibody (GL1) was purchased from PharMingen. PE-coupled CD40 antibody (3/23) was obtained from Serotec. Mouse monoclonal YAe is directed against I-Ab associated with Eα (Drα)52-68 peptide (Murphy et al., 1989; Rudensky et al., 1991; Eastman et al., 1996) and mouse monoclonal 15G4 against I-Ab/CLIP. Rat monoclonal K553 against DM was a gift from Dr. L. Karlsson, (Scripps Research Institute, La Jolla, CA). Rabbit anti–rat IgG was obtained from Dako.
Western blot analysis and β-hexosaminidase measurements
To determine the stability of MHC II in SDS, cells were cultured for 48 h in the presence or absence of 10 μg/ml LPS and lysed in SDS sample buffer. Samples of the lysates were incubated either at room temperature for 10 min at 37°C, 5 min at 60°C, or 5 min at 100°C and analyzed by Western blotting according to standard procedures using polyclonal anti–β-MHC II (see above) and ECL detection.
To determine the secretion of β-hexosaminidase, cells were cultured during the last 18, 8, 4, or 2 h in fresh medium in the presence or absence of 10 μg/ml LPS. The media were harvested simultaneously and supplemented with 1% Triton X-100. The cells were lysed in medium containing 1% Triton X-100. Media and cell lysates were centrifuged for 1 min at 10,000 g, and samples of the supernatants were analyzed for β-hexosaminidase activity as described (Green et al., 1987). Release of β-hexosaminidase was expressed as the activity in the culture medium as a percentage of the total activity, released and intracellular, from the same culture dish.
Whole-mount immunoelectron microscopy
Whole-mount immunocytochemistry was performed principally as described (Stoorvogel et al., 1996). In short, cells were grown on gold grids carrying a formvar film and processed 3 d after seeding. Cells were incubated for 1 h in medium containing dialyzed HRP (type II, 5 mg/ml; Sigma-Aldrich) and chased either in the presence or absence of LPS for the time indicated. Cells were washed rapidly at 0°C and incubated in DAB-containing buffer. After HRP-catalyzed filling of endocytic compartments with DAB polymer, soluble cytosolic proteins were removed by permeabilizing the cells with saponin. Permeabilized cells were then fixed with 2% paraformaldehyde and 0.2% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4. Immunolabeling was performed at room temperature. In double-labeling experiments, cross labeling was excluded in control samples in which the primary antibody was omitted during the second labeling step. After labeling, cells were dehydrated in ethanol, critical point dried, and coated with a carbon film. For quantitative analysis, gold particles were counted on randomly selected vesicles, tubules, or indicated sections of tubules.
Immunoelectron microscopy on cryosections
Cells were detached from the culture plates during a short incubation in PBS/2 mM EDTA. Detached cells were pooled with nonadherent cells, washed with FCS-free medium by centrifugation, and fixed in 2% paraformaldehyde and 0.2% glutaraldehyde for 2 h. The fixative was removed and free aldehydes quenched at room temperature with 50 mM glycine in PBS. The cells were embedded in 10% gelatin and prepared for ultrathin cryosectioning and immunogold labeling (Raposo et al., 1997). Ultrathin cryosections were single immunolabeled with 10 nm protein A gold particles or double immunolabeled with 10 and 15 nm gold particles. For the overall distribution of MHC II and morphometric analysis, 2 × 10 random pictures were taken at 20× for each condition. The pictures were encoded and analyzed blindly by two independent individuals (2 × 5 pictures for each per condition) for MHC II distribution and surface densities. Surface densities of internal and limiting membranes related to the cell volume were determined using a square lattice system, according to the method of Weibel (1979). The percentage of MHC II and DM labeling on the limiting membranes of MIICs was determined in the microscope on single-labeled grids by counting the distribution of 1,000 and 500 gold particles on MIICs for each condition. To determine labeling densities of MHC II and DM vacuolar and tubular/vesicular, MIICs were photographed at a 30× magnification. Only those gold particles that were within 20 nm of a membrane were counted. Labeling densities of MHC II and DM represent the number of gold particles per membrane surface area.
Acknowledgments
The authors wish to thank R. Leckie and S. Laban for technical assistance, Prof. D. James for helpful discussions, and Dr. M. Albert for critically reading the article. R.M.C. Scriwanek and M. van Peski are gratefully acknowledged for their photographic work.
M.J. Kleijmeer is supported by grant 805-48-014 from the Nederlandse Organisatie voor Wetenschappelijk Onderzoek.
M. Kleijmeer and G. Ramm contributed equally to this work.
Footnotes
Abbreviations used in this paper: CIIV, class II vesicle; DAB, diaminobenzidine; DC, dendritic cell; Ii, invariant chain; LPS, lipopolysaccharide; MHC II, major histocompatibility complex class II; MIIC, MHC II–enriched compartment; MVB, multivesicular body.
References
- Alfonso, C., and L. Karlsson. 2000. Nonclassical MHC class II molecules. Annu. Rev. Immunol. 18:113–142. [DOI] [PubMed] [Google Scholar]
- Amigorena, S., J.R. Drake, P. Webster, and I. Mellman. 1994. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature. 369:113–120. [DOI] [PubMed] [Google Scholar]
- Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- Barois, N., F. Forquet, and J. Davoust. 1997. Selective modulation of the major histocompatibility complex class II antigen presentation pathway following B cell receptor ligation and protein kinase C activation. J. Biol. Chem. 272:3641–3647. [DOI] [PubMed] [Google Scholar]
- Barois, N., F. Forquet, and J. Davoust. 1998. Actin microfilaments control the MHC class II antigen presentation pathway in B cells. J. Cell Sci. 111:1791–1800. [DOI] [PubMed] [Google Scholar]
- Bernard, W., A. Bauer, M. Guerin, and C. Oberling. 1955. Etudeau microscope electronique de corpuscules d'aspect virusal dans les epithelions mammaires de la souris. Bull. Cancer. 13:163–171. [PubMed] [Google Scholar]
- Bhattacharya, A., M.E. Dorf, and T.A. Springer. 1981. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J. Immunol. 127:2488–2495. [PubMed] [Google Scholar]
- Blum, J.S., and P. Cresswell. 1988. Role for intracellular proteases in the processing and transport of class II HLA antigens. Proc. Natl. Acad. Sci. USA. 85:3975–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell, P. 1996. Invariant chain structure and MHC class II function. Cell. 84:505–507. [DOI] [PubMed] [Google Scholar]
- Davidson, H.W., P.A. Reid, A. Lanzavecchia, and C. Watts. 1991. Processed antigen binds to newly synthesized MHC class II molecules in antigen-specific B lymphocytes. Cell. 67:105–116. [DOI] [PubMed] [Google Scholar]
- Denzer, K., M.J. Kleijmeer, H.F.G. Heijnen, W. Stoorvogel, and H.J. Geuze. 2000. Exosome: from internal vesicle of the multivesicular body to intercellular signalling device. J. Cell Sci. 113:3365–3374. [DOI] [PubMed] [Google Scholar]
- Dieu, M.C., B. Vanbervliet, A. Vicari, J.M. Bridon, E. Oldham, S. Ait-Yahia, F. Briere, A. Zlotnik, S. Lebecque, and C. Caux. 1998. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 188:373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen, C., R.A. Bryant, A.M. Lennon-Dumenil, J.A. Villadangos, P.W. Bryant, G.P. Shi, H.A. Chapman, and H.L. Ploegh. 1999. Cathepsin S controls the trafficking and maturation of MHC class II molecules in dendritic cells. J. Cell Biol. 147:775–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman, S., M. Deftos, P.C. DeRoos, D.H. Hsu, L. Teyton, N.S. Braunstein, C.J. Hackett, and A. Rudensky. 1996. A study of complexes of class II invariant chain peptide: major histocompatibility complex class II molecules using a new complex-specific monoclonal antibody. Eur. J. Immunol. 26:385–393. [DOI] [PubMed] [Google Scholar]
- Felder, S., K. Miller, G. Moehren, A. Ullrich, J. Schlessinger, and C.R. Hopkins. 1990. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 61:623–634. [DOI] [PubMed] [Google Scholar]
- Fernandez-Borja, M., R. Wubbolts, J. Calafat, H. Janssen, N. Divecha, S. Dusseljee, and J. Neefjes. 1999. Multivesicular body morphogenesis requires phosphatidyl-inositol 3-kinase activity. Curr. Biol. 9:55–58. [DOI] [PubMed] [Google Scholar]
- Germain, R.N. 1994. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 76:287–299. [DOI] [PubMed] [Google Scholar]
- Germain, R.N., and L.R. Hendrix. 1991. MHC class II structure, occupancy and surface expression determined by post-endoplasmic reticulum antigen binding. Nature. 353:134–139. [DOI] [PubMed] [Google Scholar]
- Geuze, H.J. 1998. The role of endosomes and lysosomes in MHC class II functioning. Immunol. Today. 19:282–287. [DOI] [PubMed] [Google Scholar]
- Green, S.A., K.P. Zimmer, G. Griffiths, and I. Mellman. 1987. Kinetics of intracellular transport and sorting of lysosomal membrane and plasma membrane proteins. J. Cell Biol. 105:1227–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart, D.N. 1997. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 90:3245–3287. [PubMed] [Google Scholar]
- Hirsch, J.G., M.E. Fedorko, and Z.A. Cohn. 1968. Vesicle fusion and formation at the surface of pinocytic vacuoles in macrophages. J. Cell Biol. 38:629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba, K., S. Turley, T. Iyoda, F. Yamaide, S. Shimoyama, C. Reis e Sousa, R.N. Germain, I. Mellman, and R.M. Steinman. 2000. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J. Exp. Med. 191:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, P.E., D.A. Weber, W.P. Thayer, L.E. Westerman, and C.T. Dao. 1999. Peptide exchange in MHC molecules. Immunol. Rev. 172:229–238. [DOI] [PubMed] [Google Scholar]
- Johnstone, R.M., M. Adam, J.R. Hammond, L. Orr, and C. Turbide. 1987. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 262:9412–9420. [PubMed] [Google Scholar]
- Kleijmeer, M.J., S. Morkowski, J.M. Griffith, A.Y. Rudensky, and H.J. Geuze. 1997. Major histocompatibility complex class II compartments in human and mouse B lymphoblasts represent conventional endocytic compartments. J. Cell Biol. 139:639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats, S., C.E. Grubin, S. Eastman, P. deRoos, A. Dongre, L. Van Kaer, and A.Y. Rudensky. 1998. Invariant chain-independent function of H-2M in the formation of endogenous peptide-major histocompatibility complex class II complexes in vivo. J. Exp. Med. 187:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropshofer, H., G.J. Hammerling, and A.B. Vogt. 1999. The impact of the non-classical MHC proteins HLA-DM and HLA-DO on loading of MHC class II molecules. Immunol. Rev. 172:267–278. [DOI] [PubMed] [Google Scholar]
- Lindstedt, R., M. Liljedahl, A. Peleraux, P.A. Peterson, and L. Karlsson. 1995. The MHC class II molecule H2-M is targeted to an endosomal compartment by a tyrosine-based targeting motif. Immunity. 3:561–572. [DOI] [PubMed] [Google Scholar]
- Mellman, I., S.J. Turley, and R.M. Steinman. 1998. Antigen processing for amateurs and professionals. Trends Cell Biol. 8:231–237. [DOI] [PubMed] [Google Scholar]
- Murphy, D.B., D. Lo, S. Rath, R.L. Brinster, R.A. Flavell, A. Slanetz, and C.A. Janeway, Jr. 1989. A novel MHC class II epitope expressed in thymic medulla but not cortex. Nature. 338:765–768. [DOI] [PubMed] [Google Scholar]
- Nijman, H.W., M.J. Kleijmeer, M.A. Ossevoort, V.M. Oorschot, M.P. Vierboom, M. van de Keur, P. Kenemans, W.M. Kast, H.J. Geuze, and C.J. Melief. 1995. Antigen capture and major histocompatibility class II compartments of freshly isolated and cultured human blood dendritic cells. J. Exp. Med. 182:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi, G., M. Babst, and S.D. Emr. 1998. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell. 95:847–858. [DOI] [PubMed] [Google Scholar]
- Palade, G.E. 1955. Studies on the endoplasmic reticulum. II. Simple dispositions in cells in situ. J. Biophys. Biochem. Cytol. 1:567–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay, S.L., and G.E. Palade. 1955. The fine structure of neurons. J. Biophys. Biochem. Cytol. 1:69–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, B.T., K. Teng, C. Wu, M. Adam, and R.M. Johnstone. 1985. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 101:942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, P.J., J.J. Neefjes, V. Oorschot, H.L. Ploegh, and H.J. Geuze. 1991. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 349:669–676. [DOI] [PubMed] [Google Scholar]
- Peters, P.J., G. Raposo, J.J. Neefjes, V. Oorschot, R.L. Leijendekker, H.J. Geuze, and H.L. Ploegh. 1995. Major histocompatibility complex class II compartments in human B lymphoblastoid cells are distinct from early endosomes. J. Exp. Med. 182:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre, P., and I. Mellman. 1998. Developmental regulation of invariant chain proteolysis controls MHC class II trafficking in mouse dendritic cells. Cell. 93:1135–1145. [DOI] [PubMed] [Google Scholar]
- Pierre, P., S.J. Turley, E. Gatti, M. Hull, J. Meltzer, A. Mirza, K. Inaba, R.M. Steinman, and I. Mellman. 1997. a. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 388:787–792. [DOI] [PubMed] [Google Scholar]
- Pierre, P., S.J. Turley, J. Meltzer, A. Mirza, R. Steinman, and I. Mellman. 1997. b. Localization and intracellular transport of MHC class II molecules in bone marrow-derived dendritic cells. Adv. Exp. Med. Biol. 417:179–182. [DOI] [PubMed] [Google Scholar]
- Pierre, P., I. Shachar, D. Matza, E. Gatti, R.A. Flavell, and I. Mellman. 2000. Invariant chain controls H2-M proteolysis in mouse splenocytes and dendritic cells. J. Exp. Med. 191:1057–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond, L., and C. Watts. 1999. Functional early endosomes are required for maturation of major histocompatibility complex class II molecules in human B lymphoblastoid cells. J. Biol. Chem. 274:18049–18054. [DOI] [PubMed] [Google Scholar]
- Ramm, G., L. Pond, C. Watts, and W. Stoorvogel. 2000. Clathrin-coated lattices and buds on MHC class II compartments do not selectively recruit mature MHC-II. J. Cell Sci. 113:303–313. [DOI] [PubMed] [Google Scholar]
- Raposo, G., H.W. Nijman, W. Stoorvogel, R. Leijendekker, C.V. Harding, C.J. Melief, and H.J. Geuze. 1996. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183:1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo, G., M.J. Kleijmeer, G. Posthuma, J.W. Slot, and H.J. Geuze. 1997. Immunogold labeling of ultrathin cryosections: application in immunology. Weirs Handbook of Experimental Immunology. L.A. Herzenberg, D.M. Weir, L.A. Herzenberg, and C. Blackwell, editors. Blackwell Science, Inc., Malden, MA. 208.1–208.11.
- Regnault, A., D. Lankar, V. Lacabanne, A. Rodriguez, C. Thery, M. Rescigno, T. Saito, S. Verbeek, C. Bonnerot, P. Ricciardi-Castagnoli, et al. 1999. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfrew, C.A., and A.L. Hubbard. 1991. Sequential processing of epidermal growth factor in early and late endosomes of rat liver. J. Biol. Chem. 266:4348–4356. [PubMed] [Google Scholar]
- Rescigno, M., C. Winzler, D. Delia, C. Mutini, M. Lutz, and P. Ricciardi-Castagnoli. 1997. Dendritic cell maturation is required for initiation of the immune response. J. Leukoc. Biol. 61:415–421. [PubMed] [Google Scholar]
- Rescigno, M., S. Citterio, C. Thery, M. Rittig, D. Medaglini, G. Pozzi, S. Amigorena, and P. Ricciardi-Castagnoli. 1998. Bacteria-induced neo-biosynthesis, stabilization, and surface expression of functional class I molecules in mouse dendritic cells. Proc. Natl. Acad. Sci. USA. 95:5229–5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese, R.J., P.R. Wolf, D. Bromme, L.R. Natkin, J.A. Villadangos, H.L. Ploegh, and H.A. Chapman. 1996. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 4:357–366. [DOI] [PubMed] [Google Scholar]
- Robinson, J.M., and M.J. Karnovsky. 1991. Rapid-freezing cytochemistry: preservation of tubular lysosomes and enzyme activity. J. Histochem. Cytochem. 39:787–792. [DOI] [PubMed] [Google Scholar]
- Roche, P.A., and P. Cresswell. 1991. Proteolysis of the class II-associated invariant chain generates a peptide binding site in intracellular HLA-DR molecules. Proc. Natl. Acad. Sci. USA. 88:3150–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudensky, A., P. Preston-Hurlburt, S.C. Hong, A. Barlow, and C.A. Janeway, Jr. 1991. Sequence analysis of peptides bound to MHC class II molecules. Nature. 353:622–627. [DOI] [PubMed] [Google Scholar]
- Sallusto, F., M. Cella, C. Danieli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo, J.R., and K.R. Porter. 1959. An electron microscope study of the rat ovum. J. Biophys. Biochem Cytol. 5:327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel, W., V. Oorschot, and H.J. Geuze. 1996. A novel class of clathrin-coated vesicles budding from endosomes. J. Cell Biol. 132:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, J., A. Bushnell, and S.C. Silverstein. 1987. Tubular lysosome morphology and distribution within macrophages depend on the integrity of cytoplasmic microtubules. Proc. Natl. Acad. Sci. USA. 84:1921–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, H.L., and J.G. Cyster. 1999. Chemokine up-regulation and activated T cell attraction by maturing dendritic cells. Science. 284:819–822. [DOI] [PubMed] [Google Scholar]
- Thery, C., V. Brachet, A. Regnault, M. Rescigno, P. Ricciardi-Castagnoli, C. Bonnerot, and S. Amigorena. 1998. MHC class II transport from lysosomal compartments to the cell surface is determined by stable peptide binding, but not by the cytosolic domains of the alpha- and beta-chains. J. Immunol. 161:2106–2113. [PubMed] [Google Scholar]
- Thery, C., A. Regnault, J. Garin, J. Wolfers, L. Zitvogel, P. Ricciardi-Castagnoli, G. Raposo, and S. Amigorena. 1999. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J. Cell Biol. 147:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley, S.J., K. Inaba, W.S. Garrett, M. Ebersold, J. Unternaehrer, R.M. Steinman, and I. Mellman. 2000. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 288:522–527. [DOI] [PubMed] [Google Scholar]
- Villadangos, J.A., C. Driessen, G.P. Shi, H.A. Chapman, and H.L. Ploegh. 2000. Early endosomal maturation of MHC class II molecules independently of cysteine proteases and H-2DM. EMBO J. 19:882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts, C. 1997. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu. Rev. Immunol. 15:821–850. [DOI] [PubMed] [Google Scholar]
- Weibel, E.R. 1979. Stereological Methods. Academic Press, Inc., New York. 416 pp.
- Winzler, C., P. Rovere, M. Rescigno, F. Granucci, G. Penna, L. Adorini, V.S. Zimmermann, J. Davoust, and P. Ricciardi-Castagnoli. 1997. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J. Exp. Med. 185:317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, E. 1955. The fine structure of the renal glomerulus of the mouse. J. Biophys. Biochem. Cytol. 1:551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]