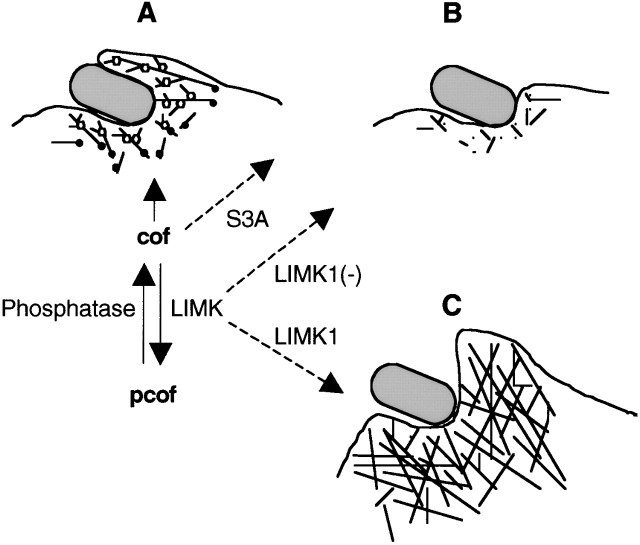
Figure 9.
Possible role of the cofilin phosphocycle in InlB- induced phagocytosis. (A) Interaction of InlB with its receptors triggers actin polymerization and formation of the phagocytic cup. This step involves recruitment of (1) the Arp2/3 complex (open circles), which promotes actin nucleation and branching of the filaments, and (2) ADF/cofilin (closed circles), which stimulates actin dynamics by severing actin filaments, creating new ends that allow actin polymerization, and by depolymerizing actin filaments, supplying new actin monomers. LIMK recruited at this step could prevent excessive depolymerization of the filaments by partly inactivating cofilin. A phosphatase could reactivate cofilin, which would finally accumulate on the filaments, thereby increasing its activity and facilitating the disruption of the F-actin network in the phagocytic cup. (B) Inhibition of endogenous LIMK by expressing the dominant negative LIMK1− or expression of the nonphophorylatable S3A mutant would lead to an excessive activity of cofilin, resulting in increased filament disassembly and abortion of the actin cup formation. (C) Overexpressing LIMK1 would increase cofilin phosphorylation, leading to the inhibition of its actin depolymerizing activity and inducing the formation of a rigid structure beneath the entering particles made of a thick filament network. These events would prevent the engulfment of the particle into the cell, resulting in the inhibition of the phagocytic cup closure.