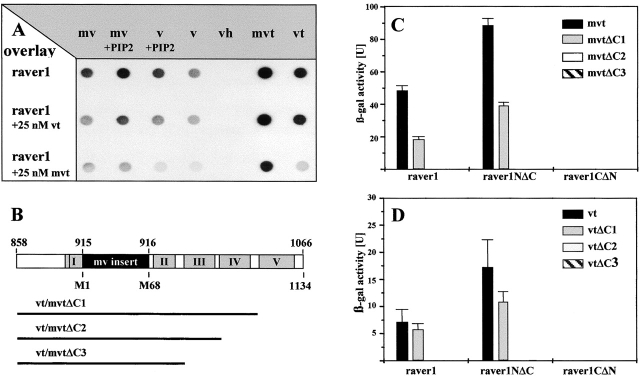
Figure 3.
Raver1 binds to the tail domain of vinculin/metavinculin. (A) Overlay spots obtained with [35S]methionine-labeled raver1 on immobilized chicken metavinculin (mv), PIP2-activated metavinculin (mv + PIP2), activated vinculin (v + PIP2), vinculin (v), vinculin head domain (vh), recombinant murine mvt (mvt), and vt. Binding is enhanced by activation of v/mv with PIP2 and is reduced in the presence of soluble vt (raver1 + 25 nM vt) or mvt (raver1 + 25 nM mvt). Some binding was also noted for nonactivated v/mv, which may be due to partial conformational changes upon immobilization to the membrane. (B) A schematic representation of vt/mvt and deletion fragments used for two-hybrid analysis shown in C and D. Highlighted areas comprise the metavinculin insert (black box) and the five α-helices within the vt domain (grey boxes numbered according to Bakolitsa et al., 1999). (C and D) Raver1–vt/mvt interaction assayed by β-galactosidase activity in a yeast two-hybrid liquid assay. Samples were analyzed in three independent experiments, and means and SD are shown. Note that binding of mvt and vt to the NH2-terminal portion of raver1 (raver1NΔC, amino acids 1–441) involves α-helices IV and V of vt and the NH2-terminal portion of raver1 (raver1NΔC, amino acids 442–748).