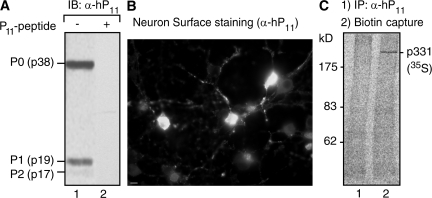
Figure 1.
Affinity-purified anti-P antibodies recognize a cell-surface protein of high molecular mass (p331) in neurons. (A) Immunoblot (IB) with affinity-purified anti-P antibodies (α-hP11) shows reaction with the P ribosomal proteins P0 (38 kD), P1 (19 kD), and P2 (17 kD; lane 1), which is blocked by the P peptide (lane 2). (B) Anti-P staining of the neuronal surface. Brain cortical primary culture cells were incubated with α-hP11 antibody at 4°C, and fixed and incubated with secondary FITC–anti–human antibody. Some neurons show intense surface staining. Bar, 10 μm. (C) Detection of anti-P target at the surface of brain cortical neurons. Neurons in primary culture were metabolically labeled for 16 h with 100 μCi [35S]methionine-cysteine, biotinylated at 4°C, and subjected to immunoprecipitation with either control P (−) serum from an SLE patient (lane 1) or α-hP11 antibodies (lane 2). The immunoprecipitated proteins were separated from the sepharose beads by heating in SDS buffer, and the biotinylated proteins were precipitated with immobilized neutravidin protein. A single high molecular mass protein (p331) is detected.