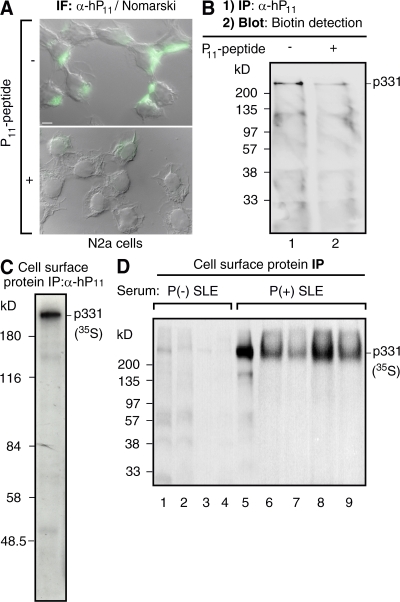
Figure 2.
Antibodies against ribosomal P epitope recognize p331 in the surface of N2a cells. (A) Anti-P indirect immunofluorescence in intact neuroblastoma N2a cells. The cell-surface staining, superimposed over images of Nomarski interference microscopy, is asymmetrically distributed and is displaced by the P peptide. Bar, 10 μm. (B) Biotinylation assays detect p331 as the cell-surface P epitope–bearing protein. Intact N2a cells were biotinylated at 4°C, lysed, and subjected to immunoprecipitation with α-hP11 antibodies either in the absence (lane 1) or presence (lane 2) of P peptide. The biotinylated proteins, resolved by SDS-PAGE, were detected in the blot with horseradish peroxidase–streptavidin. (C and D) Direct immunoprecipitation of cell-surface p331 anti-P target. N2a cells metabolically labeled for 16 h with 100 μCi [35S]methionine-cysteine were incubated intact, at 4°C, with either α-hP11 (C) or sera from lupus patients (D), including anti-P (−; lanes 1–4) or anti-P (+) with psychosis (lane 5) or without NP-SLE (lanes 6–9). The cells were lysed, and the immunocomplexes formed at the cell surface were precipitated with protein A–sepharose, resolved by SDS-PAGE, and visualized by fluorography, showing only the p331 protein.