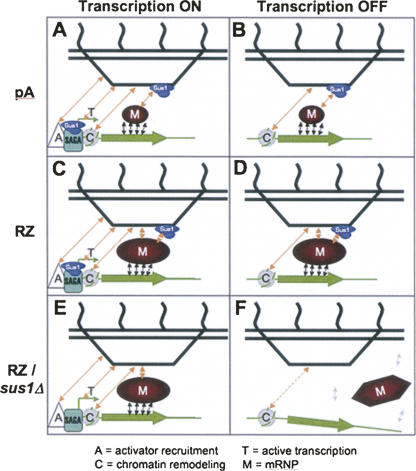
FIGURE 4.
A model that integrates the multiple interactions between the activated GAL genes (green), non-nascent mRNP pools (red), and nuclear pores (black), emphasizing the dual role of the Sac3-Thp1-Sus1-Cdc31 complex (blue) at the site of transcription as well as at the nuclear periphery. (A) In WT cells, perinuclear repositioning of the GAL-GFP-pA locus occurs via a combined action of multiple mechanisms including transcriptional activator (A)-dependent recruitment as well as transcription (T)-, chromatin (C)-, and mRNP (M)-mediated capture and retention. (B) Upon shutoff of transcription, persistent chromatin- and mRNP-mediated retention prevents the immediate departure of the GAL-GFP-pA locus from the nuclear rim. (C) The abnormal and large GAL-GFP-RZ mRNP pool produced when mRNA 3′-end processing is bypassed by RZ makes additional contacts at the nuclear pore, which are not Sac3-Thp1-Sus1-Cdc31 complex-dependent, and hence is strongly retained at the nuclear rim. (D) Upon transcriptional shutoff, the synergy of these additional contacts with the NPC, acting together with the Sac3-Thp1-Sus1-Cdc31 complex-dependent and chromatin-dependent contacts, allows the GAL-GFP-RZ mRNP to persist at the nuclear periphery longer than GAL-GFP-pA. (E) Even in the absence of the Sac3-Thp1-Sus1-Cdc31 complex at the NPC, the synergy of the activator-mediated contacts, ongoing transcription, chromatin-mediated and the Sac3-Thp1-Sus1-Cdc31 complex-independent interactions of the exaggerated GAL-GFP-RZ mRNP with the NPC support a modest degree of peripheral tethering. (F) Upon transcriptional shutoff, this modest tethering fails immediately, while the aberrant mRNP remodeling (illustrated by the change in shape of the mRNP pool) and/or compromised export of the GAL-GFP-RZ mRNP in the absence of Sac3-Thp1-Sus1-Cdc31 complex causes its separation from the gene.