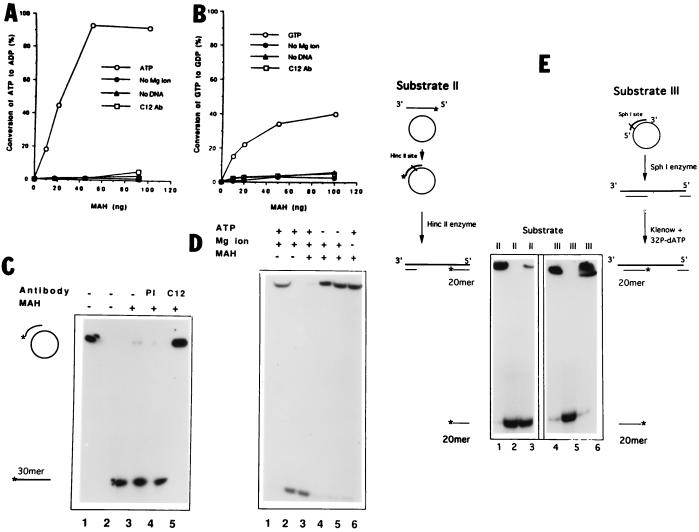
Figure 2.
(A and B) Purified MAH presents NTPase activity. MAH can hydrolyze both ATP (A) and GTP (B) in a DNA and Mg ion-dependent manner. Increasing concentrations of MAH were combined with 1 mM [2,8-3H]ATP or [2,8-3H]GTP at 30°C for 30 min. The reaction products were separated by thin layer chromatography, and the converted nucleotides were determined with autoradiography. The open circles indicate ATP or GTP hydrolysis in the presence of salmon sperm DNA and Mg ions. (C) MAH unwinds partially double-stranded DNA. Substrate I was created from circular M13 mp18 single-stranded plasmid DNA annealed with a 30-mer labeled oligonucleotide. Lanes: 1, native substrate I; 2, heat denatured substrate I without MAH protein (negative and positive controls, respectively); 3–5, 30 fmol substrate I combined with 40 ng MAH; 4–5, preincubation of MAH with preimmune serum (PI) or C12 antibody (C12). (D) MAH unwinding activity is ATP and Mg ion dependent. Lanes: 1 and 2, as in C; 3, MAH was combined with substrate I in the presence of Mg ion and ATP; 4 and 5, ATP was replaced with 1 or 2 mM ATP-γS, respectively; 6, Mg ions were omitted from the reaction mixture. (E) Polarity of MAH unwinding activity. Substrates II and III were created as described in Materials and Methods, with 5′- or 3′-end labeled short duplexes, respectively. MAH (40 ng) was combined with either substrate II (lane 3) or III (lane 6). Lanes: 1 and 4, native substrates as negative controls; 2 and 5, heat denatured substrates as positive controls.