Abstract
A library of 2,N6-disubstituted adenosine analogs was synthesized and the analogs were tested for their antiprotozoal activities. It was found that 2-methoxy and 2-histamino and N6-m-iodobenzyl substitutions generally produced analogs with low levels of antiprotozoal activity. The best antiplasmodial activity was achieved with large aromatic substitutions, such as N6-2,2-diphenylethyl and naphthylmethyl, which could indicate a mechanism of action through aromatic stacking with heme in the digestive vacuole of Plasmodium spp. The activities against Trypanosoma cruzi trypomastigotes and Leishmania donovani amastigotes were generally low; but several analogs, particularly those with cyclopentylamino substitutions, displayed potent activities against Trypanosoma brucei rhodesiense and T. b. brucei bloodstream forms in vitro. The most active were 2-cyclopentylamino-N6-cyclopentyladenosine (compound NA42) and 2-cyclopentylamino-N6-cyclopentyladenine (compound NA134), with the nucleobase an order of magnitude more potent than the nucleoside, at 26 ± 4 nM. It was determined that the mode of action of these purines was trypanostatic, with the compounds becoming trypanocidal only at much higher concentrations. Those 2,N6-disubstituted purines tested for their effects on purine transport in T. b. brucei displayed at best a moderate affinity for the transporters. It is highly probable that the large hydrophobic substitutions, which bestow high calculated octanol-water coefficient values on the analogs, allow them to diffuse across the membrane. Consistent with this view, the analogs were as effective against a T. b. brucei strain lacking the P2 nucleoside transporter as they were against the parental strain. As the analogs were not toxic to human cell lines, the purine analogs are likely to act on a trypanosome-specific target.
Tropical diseases caused by protozoan parasites, including Chagas' disease, leishmaniasis, malaria, and human African trypanosomiasis (HAT; or sleeping sickness), are a daily threat to 40% of the world's population and together are responsible for up to 3 million deaths per year (37). Rapidly growing resistance to the common drugs (8, 12) and the need for their often burdensome parenteral administration, often combined with serious adverse effects, demand the urgent development of safer and more effective drugs.
One feature common to all protozoan parasites that is fundamentally different from mammals is that these pathogens lack the capability of synthesizing purines de novo and therefore are solely dependent on their host for purine uptake (4). Each parasite species has a distinct and unique complement of purine transporters and salvage enzymes that enables the parasite to scavenge preformed purines from the host (10). The current antiprotozoal agents often derive their selectivity from selective accumulation by the parasite rather than the host cell (9, 22).
The selectivities and the efficacies of purine derivatives can thus be partially achieved by the cell surface transporters that mediate access to the cell, as substrate recognition by nucleobase and nucleoside transporters is strikingly different in humans and protozoa and purine salvage by the parasites is far more efficient at low substrate concentrations (10). The fact that purine analogs enter the parasites through multiple distinct transport proteins appears to prevent the onset of resistance to this class of antimetabolites (27).
By following this rationale, the use of purine derivatives for the treatment of various protozoan infections has been investigated, including leishmaniasis (1, 16), African trypanosomiasis (17, 34, 35), and malaria (18-20). In previous work we have described the synthesis of a library of di- and trisubstituted 5′-carboxamidoadenosine analogs and evaluated their antiprotozoal activities (30). Here, we report on the antiprotozoal activities of a library of 2,N6-disubstituted adenosine analogs (31). Several compounds showed promising antitrypanosomal activities, and they were further investigated for their modes of entry into Trypanosoma brucei, the parasite that causes sleeping sickness.
MATERIALS AND METHODS
Synthesis of 2,N6-disubstituted adenosine analogs.
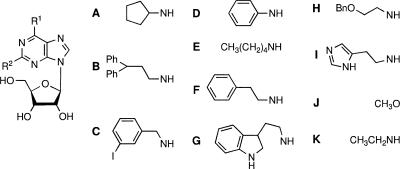
The solid-phase synthesis of compounds NA42 to NA61 has been described previously (31). Compounds NA111 to NA126 were synthesized by the same method and were obtained at a 38 to 71% yield and at >94% purity, as determined by high-pressure liquid chromatography. The synthesis of 2-cyclopropylamino-N6-cyclopentyladenine (compound NA134) will be described elsewhere. Nuclear magnetic resonance data and accurate mass measurements can be found in the supplemental material.
Cytotoxicity and antiprotozoal activity.
Evaluation of the compounds for their cytotoxicities for L6 mouse fibroblast cells and for their activities against the chloroquine- and pyrimethamine-resistant line Plasmodium falciparum K1, Trypanosoma brucei rhodesiense strain STIB 900, T. b. brucei strain BS221, the tbat1−/− clone derived from BS221 (23), trypomastigotes of Trypanosoma cruzi strain Tulahuen C4, and amastigotes of Leishmania donovani strain MHOM-ET-67/L82 in primary mouse macrophages was performed at the Swiss Tropical Institute, exactly as described previously (24, 30). Additional drug sensitivity assays with culture-adapted bloodstream T. b. brucei s427, Leishmania major promastigotes, and Leishmania mexicana amastigotes were performed at the University of Glasgow, as described previously (1, 26, 36). Melarsoprol, pentamidine, and DB75 (furamidine) are antitrypanosomal drugs; and chloroquine, mefloquine, and artemisinin are antimalarial drugs. These compounds were used as positive controls in the antiprotozoal drug assays. Cytotoxicity against human embryonic kidney (HEK) cell strain 293T was assayed as follows. HEK 293 cells were seeded in 96-well microtiter plates at 3 × 104 cells per well in 100 μl of Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% newborn bovine serum (Invitrogen), 2 mM l-glutamax (Gibco), and 1% penicillin-streptomycin (Gibco). The plate was incubated at 37°C under a 5% CO2 atmosphere for 3 h to allow the cells to adhere to the bottom of the well. Subsequently, 100 μl of medium containing serial drug dilutions covering a range of concentrations from 500 to 0.24 μM was added and the plate was incubated for another 16 h. Negative controls were obtained by using medium without test compound, whereas positive controls included a dilution series of phenylarsine oxide (Sigma). Finally, 20 μl of Alamar blue solution (12.5 mg resazurin [Sigma] dissolved in 100 ml distilled water and filter sterilized) was added to each well, and after a further 24 h of incubation, fluorescence was determined in a Perkin-Elmer Life Sciences LS55B fluorimeter (λexcitation = 530 nm, λemission = 590 nm). Data were analyzed by using GraphPad Prism software. The 50% inhibitory concentrations (IC50s) were determined in at least three independent experiments for each drug.
Purine transport studies.
Uptake assays for bloodstream-form T. b. brucei P1 and P2 nucleoside transporters and the T. b. brucei TbH2 nucleobase transporter were performed exactly as described previously (11, 36). Radiolabeled purines ([3H]adenosine [16 Ci/mmol] and [3H]hypoxanthine [28 Ci/mmol]) were obtained from Amersham Biosciences, United Kingdom. The hydrophobicities of selected adenosine analogs are presented as their calculated octanol-water coefficient (c-log P), and c-log P values were calculated by using the ChemDraw Ultra software package (CambridgeSoft).
RESULTS
Antiprotozoal screening.
The compounds were tested in vitro against chloroquine- and pyrimethamine-resistant strain Plasmodium falciparum K1 and against three trypanosomatids, Trypanosoma brucei rhodesiense (bloodstream forms; strain STIB 900), Trypanosoma cruzi (trypomastigotes in vitro; strain Tulahuen C4), and Leishmania donovani (amastigotes in mouse macrophages; strain MHOM-ET-67/L82). Most adenosine analogs did not display IC50 values below 30 μM against the South American parasites T. cruzi and L. donovani (data not shown) and, accordingly, were considered inactive against these parasites. The activities against P. falciparum and T. b. rhodesiense are listed in Table 1.
TABLE 1.
Antiprotozoal activities of 2,N6-disubstituted adenosine analogsa
IC50 values were determined in duplicate. Ph, phenyl.
b ND, not determined.
Generally, the 2-methoxy and the 2-histamino derivatives were inactive as antiprotozoal agents. Only 2-histamino-N6-diphenylethyladenosine (compound NA50) displayed moderate antimalarial activity, with an IC50 value of 8.17 μM. With the exception of the 2-cyclopentylamino derivative (compound NA52), the N6-m-iodobenzyl adenosine analogs did not manifest distinct antiprotozoal activities; the 2-cyclopentylamino derivative (compound NA52) showed a moderate inhibitory effect on T. b. rhodesiense growth, with an IC50 value of 5.44 μM. Some N6-phenyl analogs (compounds NA57 to NA59) demonstrated moderate antiprotozoal activities, and the 2-cyclopentylamino derivative (compound NA57) had significant trypanocidal action. Apparently, substitution of the 2 position of the purine ring with a cyclopentylamino group offered promising antitrypanosomal activity. All 2-cyclopentyl derivatives (compounds NA42, NA47, NA52, and NA57) displayed activities against T. b. rhodesiense at low micromolar concentrations, and the IC50 value of the biscyclopentyl adenosine analog (compound NA42) was determined to be submicromolar (0.40 μM). Although the N6-diphenylethyl analogs (compounds NA47 to NA51) did not show significant trypanocidal activities, they revealed antimalarial activities at low micromolar concentrations, with the 2-tryptamino-N6-diphenylethyl analog (compounds NA48) having the lowest IC50 value (1.83 μM) against P. falciparum of all the compounds listed in Table 1.
Synthesis and screening of a follow-up library of adenosine analogs.
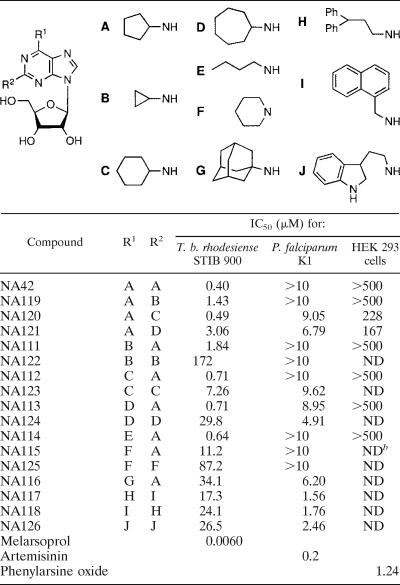
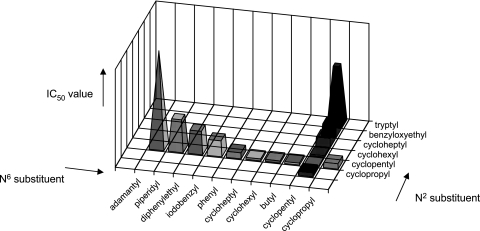
The results presented above demonstrate that substitution of the 2 position combined with substitution of the N6 position offered structural leads for antiprotozoal activity against P. falciparum and T. b. rhodesiense. For antitrypanosomal activity, the cyclopentylamino group on the 2 position combined with a small apolar substituent on N6 seems to be required (e.g., compounds NA42 and NA52). For antimalarial activity, a large aromatic moiety on N6 in combination with a large group on C-2 looks promising (e.g., compounds NA47, NA48, and NA49). With these assumptions in mind, we synthesized a follow-up library, displayed in Table 2, by making use of the solid-phase method described earlier (31). This library contains the following components: first, several cycloalkylamino-substituted adenosine analogs for examination of the relation between ring size and trypanocidal activity (compounds NA111 to NA113 and NA119 to NA124); second, derivatives with an open chain alkyl group on N6 combined with a cycloalkylamino group on C-2 for assessment of the need for cyclic aliphatic N6 substituents (compound NA114); third, piperidine-substituted analogs for investigation of the requirement for N2 or N6 protons (compounds NA115 and NA125); fourth, analogs with a large lipophilic bulk, such as the adamantyl moiety, on N6, which may provide information about whether lipophilicity is a cardinal factor for antitrypanosomal action (compound NA116); and finally, adenosine derivatives substituted with various aromatic substituents on C-2 and N6, which may shed light on the nature of the aromatic groups required for antiplasmodial activity (compounds NA117, NA118, and NA126). From the data in Table 1 and Table 2, a clear relationship between the ring size of the cycloalkyl substituent and antitrypanosomal capacity can be deduced. This trend is graphically represented in Fig. 1.
TABLE 2.
Antiprotozoal activities of a follow-up library of 2,N6-disubstituted adenosine analogsa
IC50 values were determined in duplicate. Ph, phenyl.
b ND, not determined.
FIG. 1.
Optimization of N6 and N2 substituents for trypanocidal activity. Substituents are arranged by size.
For both the N2 and the N6 positions, the cyclopentyl moiety appears to be optimal among the structures tested. When substituents are much larger, such as cycloheptyl or adamantyl, or when they are smaller, e.g., cyclopropyl, the activities of the compounds against T. b. rhodesiense fall off markedly. Also, when cycloalkyl groups other than cyclopentyl are present on N2 or N6, the antitrypanosomal activity is greatly reduced (see the data for compounds NA122 to NA125 in Table 2). A proton on the N6 position seems to be required for trypanocidal ability, since introduction of a piperidyl group on the purine C-6 position (compound NA115 in Table 2) results in a substantial loss of activity.
For antiplasmodial activity, the introduction of a 2,2-diphenylethyl group on the N6 position appeared to be favorable (compounds NA47, NA48, NA49, and NA117). In the first batch of adenosine derivatives, the combination with a tryptamino substituent on the 2 position furnished high antimalarial activity, with an IC50 value of 1.83 μM (Table 1, compound NA48); replacement of this 2-substituent with a naphthylmethyl group (Table 2, compound NA117) resulted in only slightly improved activity, with an IC50 value of 1.56 μM. Also, introduction of a naphthylmethyl group on the N6 position (Table 2, compound NA118) revealed antimalarial activity similar to that of NA117, with an IC50 value of 1.76 μM.
Purine transport studies in Trypanosoma brucei.
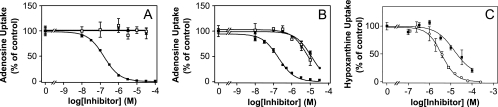
On the basis of the existing models for adenosine binding by T. b. brucei nucleoside transporters (2, 9, 11), the optimal affinity of adenosine analogs for the T. b. brucei P1 nucleoside transporter requires a riboside group with 3′ and 5′ OH groups intact, combined with N-3 and N-7 H-bond acceptors. Affinity for the P2 adenine/adenosine transporter, in contrast, requires the presence of an N6 H-bond donor in conjunction with an N-1 H-bond acceptor, an aromatic ring contributing to π-π interactions, and a group at the position corresponding to N-9, which engages in electrostatic interactions (22). The adenosine analogs generated seem to meet these general P1 and P2 recognition requirements. Therefore, two of the compounds with high trypanocidal activities, compounds NA42 and NA114, were assessed for their affinities for P1 and P2 (Fig. 2A and B, respectively). Whereas 2′-deoxyadenosine, used as a control, displayed substantial inhibitory activity on [3H]adenosine uptake, neither compound NA42 nor compound NA114 displayed an affinity for P1 and the two compounds displayed relatively low affinities for P2. Inhibition constants (Ki values) for the inhibition of [3H]adenosine uptake by P2 were determined to be 20 ± 9 μM for compound NA42 (n = 4), 9.0 ± 1.7 for compound NA114 (n = 4), and 0.23 ± 0.04 for 2′-deoxyadenosine (n = 3). The relatively low affinity for the T. b. brucei nucleoside transporters is clearly the result of steric hindrance, particularly at position 2. It should be noted that similar Ki values for inhibition of the P2 transporter by 2-substituted aminopurines have been reported, whereas for P1 the substitutions on positions 2 and N6 are both likely to contribute to steric hindrance and hence the lack of binding (2, 11).
FIG. 2.
Effects of purine analogs on P1-mediated (A) and P2-mediated (B) transport of 0.04 μM [3H]adenosine and H2-mediated transport of 0.05 μM [3H]hypoxanthine (C) by wild-type Trypanosoma brucei brucei bloodstream forms. (A and B) ▪, 2′-deoxyadenosine; □, NA42; •, NA114. (C) ▪, NA134; ○, adenine. Inhibition is expressed as a percentage of radiolabel transport in the absence of inhibitor. P1-mediated transport was assessed in the presence of 100 μM adenine and P2-mediated transport was assessed in the presence of 1 mM inosine, as described previously (11). The data points are the averages of triplicate determinations, and error bars are standard errors.
Antitrypanosomal activity against a tbat1-knockout strain.
In order to assess whether the 2,6-disubstituted adenosine analogs are internalized via the P2 transport route or primarily by passive diffusion, a selection of compounds was screened against T. b. brucei wild-type strain BS221 and the derived line T. b. brucei tbat1−/−. The latter strain displays no detectable P2 transport activity, as the tbat1 gene that encodes this transporter has been deleted by homologous recombination (23). Lipophilicity determines the probability that a compound is internalized by passive diffusion through the cell membrane. Therefore, c-log P values were calculated, as described in Materials and Methods. These data, combined with the antitrypanosomal results, are depicted in Table 3. The observed similarity in IC50 values between the tbat1+/+ and tbat1−/− strains indicates that the P2 transporter is not the main route of entry into the parasite but that diffusion is the more probable way of internalization. The calculated log P values show that most of the disubstituted adenosine analogs are indeed hydrophobic in nature and are therefore likely to traverse the cell membrane unaided.
TABLE 3.
Effects of selected 2,N6-disubstituted adenosine analogs on the ΔtbAt1 strain of T. b. bruceia
| Compound | IC50 (μM) for:
|
Resistance factor for tbat1−/−/BS221 | c-log P | |
|---|---|---|---|---|
| T. b. brucei BS221 | T. b. brucei tbat1−/− | |||
| NA42 | 0.38 | 0.20 | 0.53 | 2.52 |
| NA46 | 24.1 | 17.4 | 0.72 | 1.11 |
| NA47 | 9.28 | 4.59 | 0.49 | 4.58 |
| NA52 | 10.9 | 5.86 | 0.54 | 3.62 |
| NA57 | 1.52 | 0.79 | 0.52 | 3.02 |
| NA120 | 1.26 | 0.85 | 0.67 | 3.08 |
| NA112 | 1.46 | 0.78 | 0.53 | 3.08 |
| NA113 | 5.15 | 4.00 | 0.78 | 3.64 |
| NA114 | 0.39 | 0.29 | 0.74 | 2.63 |
| NA115 | 16.6 | 7.82 | 0.47 | 2.29 |
| NA123 | 8.53 | 5.34 | 0.62 | 3.64 |
| NA134 | NDb | ND | ND | 4.53 |
| Melarsoprol | 0.0041 | 0.0180 | 4.4 | 1.41c |
| Pentamidine | 0.0024 | 0.0060 | 2.5 | 2.79c |
| DB75 | 0.0027 | 0.0334 | 12.6 | 2.52 |
IC50 values were determined in duplicate (n = 2).
ND, not determined.
Log P value reported previously (http://chem.sis.nlm.nih.gov/).
The ribosyl moiety is not essential for antitrypanosomal activity.
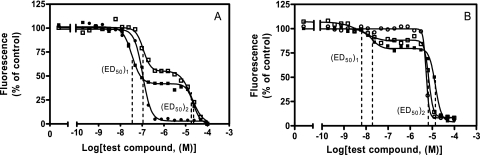
For trypanosomes, nucleobases may make more efficient drugs than their corresponding nucleosides, because purine ribonucleosides are mostly hydrolyzed in bloodstream-form T. brucei before the resulting nucleobases are converted into nucleotides by phosphoribosyltransferases in a one-step reaction (16), although T. b. brucei does encode two adenosine kinase genes (A. Lüscher and P. Mäser, personal communication). The dominant role of the phosphoribosyltransferase pathway potentially limits the incorporation of nucleosides into the nucleotide pool. Therefore, we tested whether 2,6-bis(cyclopentylamino)purine (compound NA134), which is the nucleobase analog of the most active adenosine analog, compound NA42, indeed has greater antiprotozoal activity. Figure 3 shows the effects of compounds NA42 and NA134 on T. b. brucei strain 427. As anticipated, compound NA134 showed greater antitrypanosomal activity than compound NA42 (P < 0.05). Moreover, this closer look at growth inhibition, by using the metabolism of the Alamar blue fluorescent dye as a proxy for cell number, reveals a remarkable biphasic inhibition curve, which is ascribed to a trypanostatic effect at low concentrations and a cytolytic effect at higher concentrations. The average IC50 value of compound NA42, which is depicted in Tables 1 and 3 and which was determined by the use of a different assay protocol involving a much shorter time of incubation with the reporter dye, may in fact be composed of a trypanostatic activity and a trypanocidal activity which were not separately detected by using the original screening protocol.
FIG. 3.
Growth inhibition of T. b. brucei s427 (A) and L. major promastigotes (B) by NA42 and NA134. The curves represent the results of a typical experiment. □, NA42; ▪, NA134; •, diminazene; ○, pentamidine. For T. b. brucei s427, the NA42 (ED50)1 was 0.166 ± 0.04 μM, the NA42 (ED50)2 was 15.7 ± 2.1 μM, the NA134 (ED50)1 was 0.026 ± 0.004 μM, and the NA134 (ED50)2 was 23.4 ± 0.9 μM. Diminazene was used as a positive control (IC50, 0.107 ± 0.010 μM). For L. major promastigotes, the NA42 (ED50)1 was 0.010 ± 0.002 μM, the NA42 (ED50)2 was 9.07 ± 1.07 μM, the NA134 (ED50)1 was 0.015 ± 0.003 μM, and the NA134 (ED50)2 was 15.1 ± 4.6 μM. Pentamidine was used as a positive control (IC50, 3.52 ± 0.46 μM).
A similar biphasic inhibition curve was observed when we assessed compounds NA42 and NA134 for their activities against L. major promastigotes (Fig. 3B). However, these compounds did not show this biphasic effect against axenic L. mexicana amastigotes, with compound NA42 having an IC50 value of 9.01 ± 1.50 μM and compound NA134 having an IC50 value of 15.2 ± 2.0 μM (data not shown).
Given its high lipophilicity, compound NA134 is expected to enter the parasite by passive diffusion. Assessment of the inhibition of [3H]hypoxanthine uptake by the TbH2 purine nucleobase transporter by compound NA134 revealed a Ki of 33.4 ± 11.3 μM (n = 3) (Fig. 2C), indicating that translocation via purine transporters is unlikely at therapeutically relevant extracellular concentrations.
DISCUSSION
Evaluation of our library of 2,N6-disubstituted adenosine analogs showed that for antiplasmodial activity the purine skeleton requires substitution with bulky aromatic groups. Such derivatives (compounds NA48, NA117, and NA118) displayed little variation in their antimalarial activities, with IC50 values that hovered around 1.6 μM. We therefore believe that the antiplasmodial activities of these adenosine analogs in general might well be attributed to π-π interactions with heme molecules, which play a crucial role in P. falciparum metabolism (32). The classical antimalarial quinolines, quinine and chloroquine, are also believed to act via complexation to heme molecules (14, 15, 29), as are diamidines, such as pentamidine (33). Other studies by us (30) and others (20) on the antiplasmodial activities of adenosine derivatives also revealed that substitution of the parent adenosine structure with groups rich in π electrons favored their antimalarial activity. The targeting of the unique hemoglobin degradation pathways of Plasmodium would also be consistent with the relatively low levels of activity of these aromatically substituted compounds against Trypanosoma and Leishmania spp. (Table 1). Nevertheless, it cannot be excluded that targets other than heme complexation are affected, including a variety of nucleotide-dependent enzymes, such as cyclin-dependent kinases (13, 19) and the parasite's nucleoside salvage pathways (10).
Late-stage trypanosomiasis, when parasites have invaded the central nervous system, is fatal if it is left untreated, and unfortunately, the available treatment for this cerebral parasitemia is highly unsatisfactory. Eflornithine, the sole drug developed in recent decades, is effective only against late-stage West African sleeping sickness and is very expensive (28). Considering the rapidly growing rates of resistance to melarsoprol (7, 21), the sole drug effective against East African late-stage HAT caused by T. b. rhodesiense, and the high cerebrotoxicity of this arsenical compound (3), there is an urgent need for the development of safe and efficacious drugs for the treatment of late-stage HAT.
To combat the neuropathological form of the disease, drugs will have to cross the blood-brain barrier and, hence, will need to be lipophilic in character. The most active antitrypanosomal compounds resulting from our screen, nucleoside derivative NA42 and its nucleobase analog, compound NA134, in fact seem to meet that requirement, given their high c-log P values. The relatively low affinity for P1 and P2 transporters and for the H2 transporter of compounds NA42 and NA134, respectively, combined with their similar activities against the tbat1−/− and parental strains, reveals that these compounds must enter the parasite by passive diffusion across the cell membrane to cause their antiprotozoal effects. Accordingly, the higher hydrophobicity of compound NA134 compared with that of NA42 may well contribute to the higher trypanocidal activity of compound NA134. Other reasons for its higher activity might be that upon metabolism within the parasite, compound NA134 is already a substrate for phosphoribosyltransferases, whereas the ribosyl group of compound NA42 would first have to be hydrolyzed before entry into the nucleotide pool (16). Nevertheless, there is the possibility that these two molecules exert their antiprotozoal activities in their unmetabolized forms, for instance, by inhibiting certain ATP- or NAD(P)H-dependent enzymes, such as the kinases or enzymes involved in glycolysis. Over the past few years several studies that described the rational targeting of glycolytic enzymes by adenosine derivatives have appeared (6). Although various inhibitors of T. brucei and T. cruzi glyceraldehyde-3-phosphate dehydrogenase (6) and T. brucei phosphoglycerate kinase (5) have been identified, these compounds displayed antitrypanosomal activities only in the low micromolar range; i.e., they were 1 to 2 orders of magnitude less effective than compounds NA42 and NA134.
Current antiprotozoal agents often derive their selectivity from selective accumulation by the parasite rather than the host cell (9). Although the 2,N6-disubstituted adenosine derivatives were, in principle, designed to be selectively accumulated by the parasite through active transport by the protozoan nucleoside and nucleobase carriers, they appear to have little affinity for the trypanosomal transporters. Nonetheless, the absence of mammalian cytotoxicity of compounds NA42 and NA134 indicates that their selectivity may come from intracellular biochemical or metabolic differences between parasite and host cells. Alternatively, the high tolerance of mammalian cells toward these nucleoside and nucleobase derivatives may be attributed to efficient mammalian drug efflux pumps which are capable of transporting lipophilic nucleoside analogs from mammalian cells (10). Their beneficial in vitro profiles make compounds NA42 and NA134 good candidates for further in vivo evaluation as potential therapeutics against African trypanosomiasis.
Supplementary Material
Acknowledgments
Nasser El-Sabbagh and Hasan Ibrahim are gratefully acknowledged for their expert technical assistance.
The Netherlands Organization for Scientific Research (NWO) is acknowledged for its financial support (to B.R.).
Footnotes
Published ahead of print on 13 August 2007.
Supplemental material for the article may be found at http://aac.asm.org/.
REFERENCES
- 1.Al Salabi, M. I., and H. P. De Koning. 2005. Purine nucleobase transport in amastigotes of Leishmania mexicana: involvement in allopurinol uptake. Antimicrob. Agents Chemother. 49:3682-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Salabi, M. I., L. J. Wallace, A. Luscher, P. Maser, D. Candlish, B. Rodenko, M. K. Gould, I. Jabeen, S. N. Ajith, and H. P. De Koning. 2007. Molecular interactions underlying the unusually high adenosine affinity of a novel Trypanosoma brucei nucleoside transporter. Mol. Pharmacol. 71:921-929. [DOI] [PubMed] [Google Scholar]
- 3.Barrett, M. P., R. J. Burchmore, A. Stich, J. O. Lazzari, A. C. Frasch, J. J. Cazzulo, and S. Krishna. 2003. The trypanosomiases. Lancet 362:1469-1480. [DOI] [PubMed] [Google Scholar]
- 4.Berens, R. L., E. C. Krug, and J. J. Marr. 1995. Purine and pyrimidine metabolism, p. 89-117. In J. J. Marr and M. Muller (ed.), Biochemistry and molecular biology of parasites. Academic Press, Inc., New York, NY.
- 5.Bressi, J. C., J. Choe, M. T. Hough, F. S. Buckner, W. C. Van Voorhis, C. L. Verlinde, W. G. Hol, and M. H. Gelb. 2000. Adenosine analogues as inhibitors of Trypanosoma brucei phosphoglycerate kinase: elucidation of a novel binding mode for a 2-amino-N6-substituted adenosine. J. Med. Chem. 43:4135-4150. [DOI] [PubMed] [Google Scholar]
- 6.Bressi, J. C., C. L. Verlinde, A. M. Aronov, M. L. Shaw, S. S. Shin, L. N. Nguyen, S. Suresh, F. S. Buckner, W. C. Van Voorhis, I. D. Kuntz, W. G. Hol, and M. H. Gelb. 2001. Adenosine analogues as selective inhibitors of glyceraldehyde-3-phosphate dehydrogenase of Trypanosomatidae via structure-based drug design. J. Med. Chem. 44:2080-2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brun, R., R. Schumacher, C. Schmid, C. Kunz, and C. Burri. 2001. The phenomenon of treatment failures in human African trypanosomiasis. Trop. Med. Int. Health 6:906-914. [DOI] [PubMed] [Google Scholar]
- 8.Croft, S. L. 2001. Monitoring drug resistance in leishmaniasis. Trop. Med. Int. Health 6:899-905. [DOI] [PubMed] [Google Scholar]
- 9.De Koning, H. P. 2001. Transporters in African trypanosomes: role in drug action and resistance. Int. J. Parasitol. 31:512-522. [DOI] [PubMed] [Google Scholar]
- 10.De Koning, H. P., D. J. Bridges, and R. J. Burchmore. 2005. Purine and pyrimidine transport in pathogenic protozoa: from biology to therapy. FEMS Microbiol. Rev. 29:987-1020. [DOI] [PubMed] [Google Scholar]
- 11.De Koning, H. P., and S. M. Jarvis. 1999. Adenosine transporters in bloodstream forms of Trypanosoma brucei brucei: substrate recognition motifs and affinity for trypanocidal drugs. Mol. Pharmacol. 56:1162-1170. [DOI] [PubMed] [Google Scholar]
- 12.Delespaux, V., and H. P. De Koning. 2007. Drugs and drug resistance in African trypanosomiasis. Drug Resist. Update 10:30-50. [DOI] [PubMed] [Google Scholar]
- 13.Doerig, C., L. Meijer, and J. C. Mottram. 2002. Protein kinases as drug targets in parasitic protozoa. Trends Parasitol. 18:366-371. [DOI] [PubMed] [Google Scholar]
- 14.Dorn, A., S. R. Vippagunta, H. Matile, C. Jaquet, J. L. Vennerstrom, and R. G. Ridley. 1998. An assessment of drug-haematin binding as a mechanism for inhibition of haematin polymerisation by quinoline antimalarials. Biochem. Pharmacol. 55:727-736. [DOI] [PubMed] [Google Scholar]
- 15.Egan, T. J., D. C. Ross, and P. A. Adams. 1994. Quinoline anti-malarial drugs inhibit spontaneous formation of beta-haematin (malaria pigment). FEBS Lett. 352:54-57. [DOI] [PubMed] [Google Scholar]
- 16.El Kouni, M. H. 2003. Potential chemotherapeutic targets in the purine metabolism of parasites. Pharmacol. Ther. 99:283-309. [DOI] [PubMed] [Google Scholar]
- 17.Gelb, M. H., and W. G. Hol. 2002. Parasitology. Drugs to combat tropical protozoan parasites. Science 297:343-344. [DOI] [PubMed] [Google Scholar]
- 18.Golisade, A., J. Wiesner, C. Herforth, H. Jomaa, and A. Link. 2002. Anti-malarial activity of N6-substituted adenosine derivatives. Part I. Bioorg. Med. Chem. 10:769-777. [DOI] [PubMed] [Google Scholar]
- 19.Harmse, L., R. van Zyl, N. Gray, P. Schultz, S. Leclerc, L. Meijer, C. Doerig, and I. Havlik. 2001. Structure-activity relationships and inhibitory effects of various purine derivatives on the in vitro growth of Plasmodium falciparum. Biochem. Pharmacol. 62:341-348. [DOI] [PubMed] [Google Scholar]
- 20.Herforth, C., J. Wiesner, S. Franke, A. Golisade, H. Jomaa, and A. Link. 2002. Antimalarial activity of N6-substituted adenosine derivatives. Part 2. J. Comb. Chem. 4:302-314. [DOI] [PubMed] [Google Scholar]
- 21.Legros, D., S. Evans, F. Maiso, J. C. Enyaru, and D. Mbulamberi. 1999. Risk factors for treatment failure after melarsoprol for Trypanosoma brucei gambiense trypanosomiasis in Uganda. Trans. R. Soc. Trop. Med. Hyg. 93:439-442. [DOI] [PubMed] [Google Scholar]
- 22.Lüscher, A., H. P. De Koning, and P. Mäser. 2007. Chemotherapeutic strategies against Trypanosoma brucei: drug targets vs. drug targeting. Curr. Pharm. Des. 13:555-567. [DOI] [PubMed] [Google Scholar]
- 23.Matovu, E., M. L. Stewart, F. Geiser, R. Brun, P. Maser, L. J. Wallace, R. J. Burchmore, J. C. Enyaru, M. P. Barrett, R. Kaminsky, T. Seebeck, and H. P. De Koning. 2003. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot. Cell 2:1003-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mbwambo, Z. H., S. Apers, M. J. Moshi, M. C. Kapingu, S. Van Miert, M. Claeys, R. Brun, P. Cos, L. Pieters, and A. Vlietinck. 2004. Anthranoid compounds with antiprotozoal activity from Vismia orientalis. Planta Med. 70:706-710. [DOI] [PubMed] [Google Scholar]
- 25.Reference deleted.
- 26.Mikus, J., and D. Steverding. 2000. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar blue. Parasitol. Int. 48:265-269. [DOI] [PubMed] [Google Scholar]
- 27.Natto, M. J., L. J. Wallace, D. Candlish, M. I. Al Salabi, S. E. Coutts, and H. P. De Koning. 2005. Trypanosoma brucei: expression of multiple purine transporters prevents the development of allopurinol resistance. Exp. Parasitol. 109:80-86. [DOI] [PubMed] [Google Scholar]
- 28.Pepin, J., and F. Milord. 1994. The treatment of human African trypanosomiasis. Adv. Parasitol. 33:1-47. [DOI] [PubMed] [Google Scholar]
- 29.Ridley, R. G., A. Dorn, S. R. Vippagunta, and J. L. Vennerstrom. 1997. Haematin (haem) polymerization and its inhibition by quinoline antimalarials. Ann. Trop. Med. Parasitol. 91:559-566. [DOI] [PubMed] [Google Scholar]
- 30.Rodenko, B., R. J. Detz, V. A. Pinas, C. Lambertucci, R. Brun, M. J. Wanner, and G. J. Koomen. 2006. Solid phase synthesis and antiprotozoal evaluation of di- and trisubstituted 5′-carboxamidoadenosine analogues. Bioorg. Med. Chem. 14:1618-1629. [DOI] [PubMed] [Google Scholar]
- 31.Rodenko, B., M. J. Wanner, and G. J. Koomen. 2002. Solid phase synthesis of C2,N-6-disubstituted adenosine analogues. J. Chem. Soc. Perkins Trans. 1:1247-1252. [Google Scholar]
- 32.Senge, M., and S. Hatscher. 2000. The malaria pigment haemozoin-a focal point of action for antimalarial drugs. Chembiochem 1:247-249. [DOI] [PubMed] [Google Scholar]
- 33.Stead, A. M. W., P. G. Bray, I. G. Edwards, H. P. De Koning, B. C. Elford, P. A. Stocks, and S. A. Ward. 2001. Diamidine compounds: selective uptake and targeting in Plasmodium falciparum. Mol. Pharmacol. 59:1298-1306. [DOI] [PubMed] [Google Scholar]
- 34.Verlinde, C. L., V. Hannaert, C. Blonski, M. Willson, J. J. Perie, L. A. Fothergill-Gilmore, F. R. Opperdoes, M. H. Gelb, W. G. Hol, and P. A. Michels. 2001. Glycolysis as a target for the design of new anti-trypanosome drugs. Drug Resist. Update 4:50-65. [DOI] [PubMed] [Google Scholar]
- 35.Wallace, L. J., D. Candlish, A. Hagos, K. L. Seley, and H. P. De Koning. 2004. Selective transport of a new class of purine antimetabolites by the protozoan parasite Trypanosoma brucei. Nucleosides Nucleotides Nucleic Acids 23:1441-1444. [DOI] [PubMed] [Google Scholar]
- 36.Wallace, L. J. M., D. Candlish, and H. P. De Koning. 2002. Different substrate recognition motifs of human and trypanosome nucleobase transporters—selective uptake of purine antimetabolites. J. Biol. Chem. 277:26149-26156. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. 2002. World health report 2002. http://www.who.int/whr/2002/en. World Health Organization, Geneva, Switzerland.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.