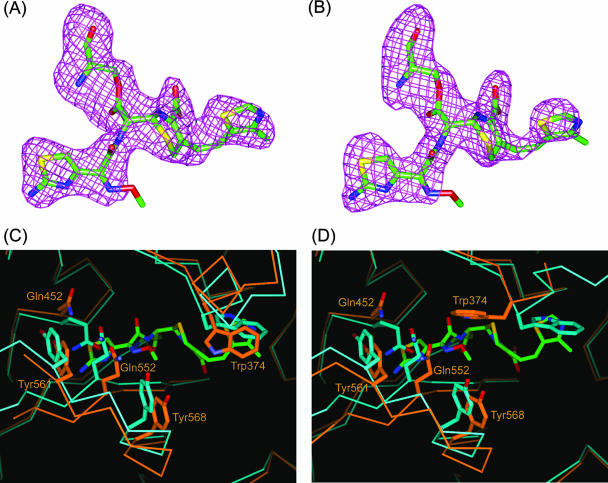
FIG. 3.
Cefditoren-PBP 2X complex structure and conformational changes. (A and B) Omit Fo-Fc difference density maps (contoured at 3 σ) covering the cefditoren moiety covalently attached to Ser337 of the trypsin-digested PBP 2X in molecules 1 and 2, respectively. Cefditoren-acylated Ser residues are shown as stick models with carbon atoms colored green, oxygens red, nitrogens blue, and sulfurs yellow. (C and D) Superposition of the active-site region from the uncomplexed (light blue) and cefditoren complex (orange) structures in molecules 1 and 2, respectively. Cefditoren and side chains of selected residues are shown as thick stick renderings. Atoms are colored as described for panel A, except that the carbon atoms of selected residues in the uncomplexed and cefditoren complex structures are colored light blue and orange, respectively. Covalent binding of cefditoren to the trypsin-digested PBP 2X requires conformational changes in the active site.