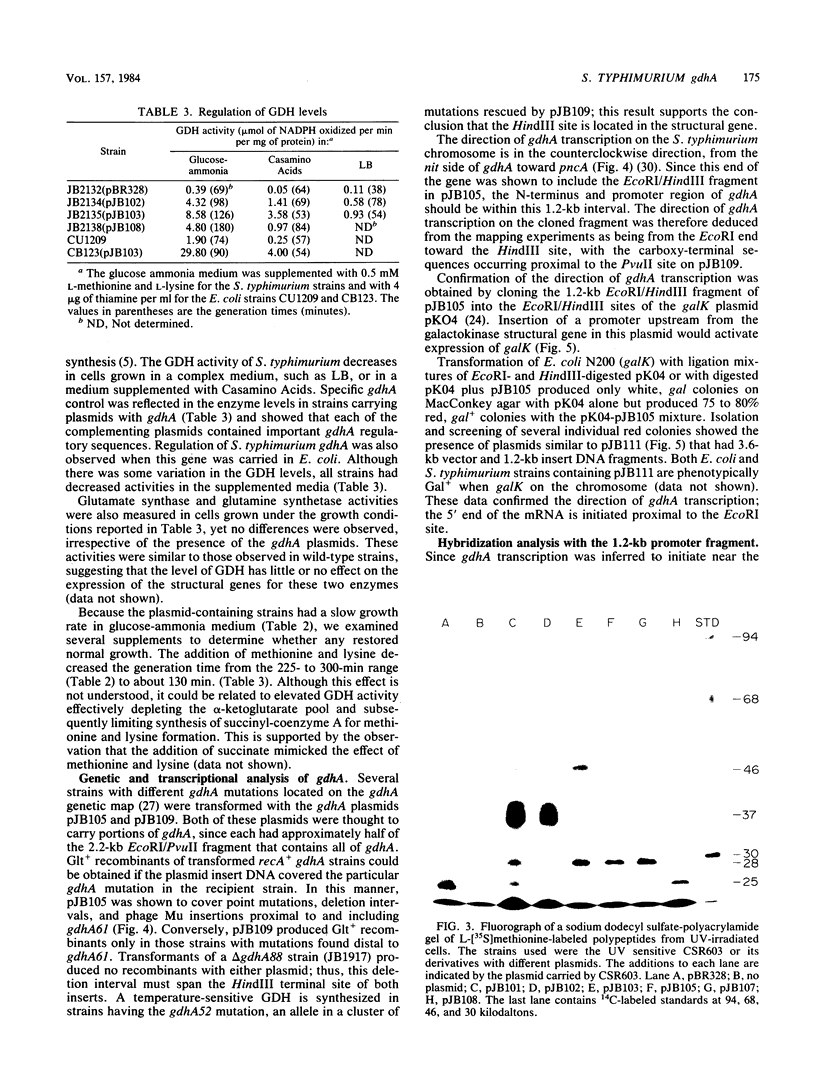
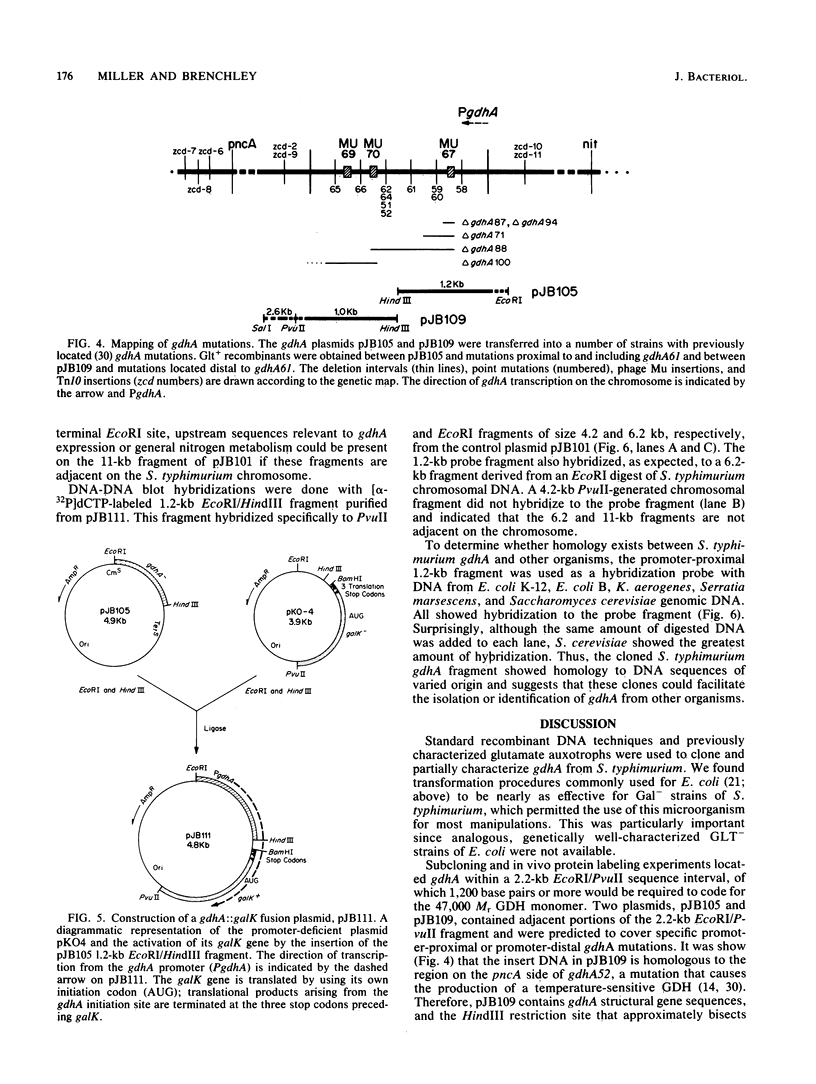
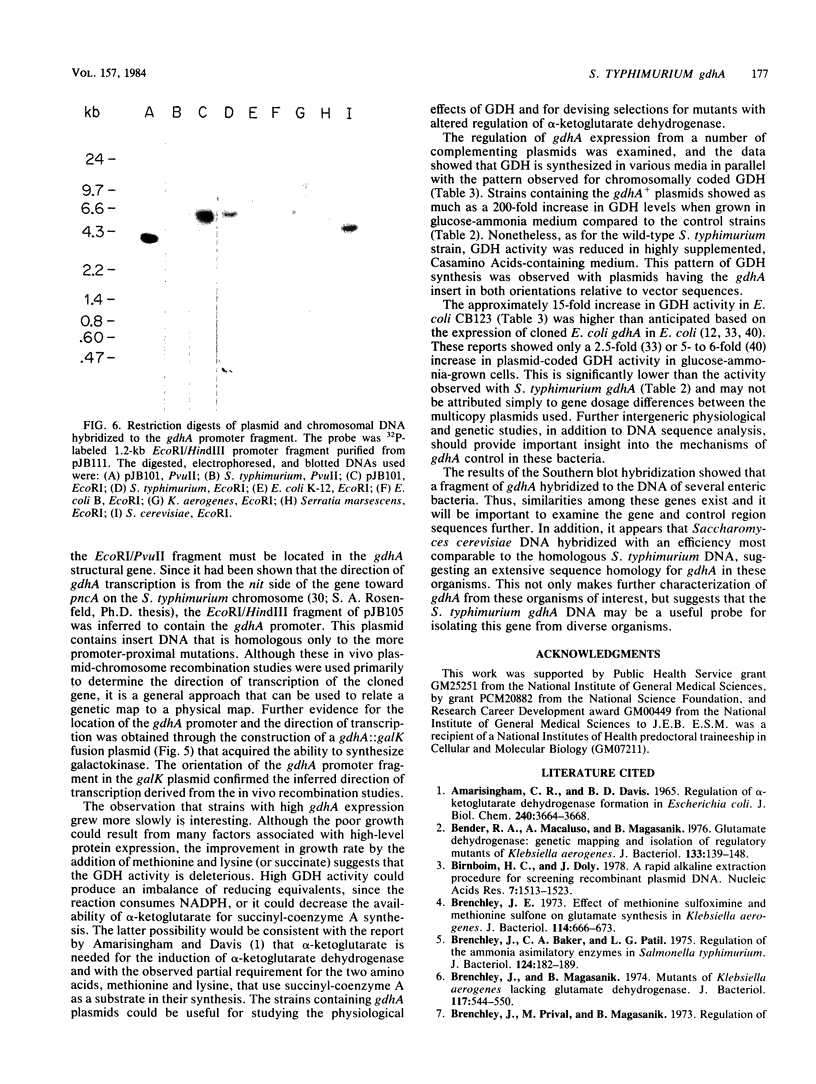
Abstract
Glutamic acid is synthesized in enteric bacteria by either glutamate dehydrogenase or by the coupled activities of glutamate synthase and glutamine synthetase. A hybrid plasmid containing a fragment of the Salmonella typhimurium chromosome cloned into pBR328 restores growth of glutamate auxotrophs of S. typhimurium and Escherichia coli strains which have mutations in the genes for glutamate dehydrogenase and glutamate synthase. A 2.2-kilobase pair region was shown by complementation analysis, enzyme activity measurements, and the maxicell protein synthesizing system to carry the entire glutamate dehydrogenase structural gene, gdhA. Glutamate dehydrogenase encoded by gdhA carried on recombinant plasmids was elevated 5- to over 100-fold in S. typhimurium or E. coli cells and was regulated in both organisms. The gdhA promoter was located by recombination studies and by the in vitro fusion to, and activation of, a promoter-deficient galK gene. Additionally, S. typhimurium gdhA DNA was shown to hybridize to single restriction fragments of chromosomes from other enteric bacteria and from Saccharomyces cerevisiae.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amarasingham C. R., Davis B. D. Regulation of alpha-ketoglutarate dehydrogenase formation in Escherichia coli. J Biol Chem. 1965 Sep;240(9):3664–3668. [PubMed] [Google Scholar]
- Bender R. A., Macaluso A., Magasanik B. Glutamate dehydrogenase: genetic mapping and isolation of regulatory mutants of Klebsiella aerogenes. J Bacteriol. 1976 Jul;127(1):141–148. doi: 10.1128/jb.127.1.141-148.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J. E., Baker C. A., Patil L. G. Regulation of the ammonia assimilatory enzymes in Salmonella typhimurium. J Bacteriol. 1975 Oct;124(1):182–189. doi: 10.1128/jb.124.1.182-189.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J. E. Effect of methionine sulfoximine and methionine sulfone on glutamate synthesis in Klebsiella aerogenes. J Bacteriol. 1973 May;114(2):666–673. doi: 10.1128/jb.114.2.666-673.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J. E., Magasanik B. Mutants of Klebsiella aerogenes lacking glutamate dehydrogenase. J Bacteriol. 1974 Feb;117(2):544–550. doi: 10.1128/jb.117.2.544-550.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broach J., Neumann C., Kustu S. Mutant strains (nit) of Salmonella typhimurium with a pleiotropic defect in nitrogen metabolism. J Bacteriol. 1976 Oct;128(1):86–98. doi: 10.1128/jb.128.1.86-98.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosloy S. D., Oishi M. The nature of the transformation process in Escherichia coli K12. Mol Gen Genet. 1973 Jul 31;124(1):1–10. doi: 10.1007/BF00267159. [DOI] [PubMed] [Google Scholar]
- Coulton J. W., Kapoor M. Purification and some properties of the glutamate dehydrogenase of Salmonella typhimurium. Can J Microbiol. 1973 Apr;19(4):427–438. doi: 10.1139/m73-071. [DOI] [PubMed] [Google Scholar]
- Covarrubias A. A., Sánchez-Pescador R., Osorio A., Bolivar F., Bastarrachea F. ColE1 hybrid plasmids containing Escherichia coli genes involved in the biosynthesis of glutamate and glutamine. Plasmid. 1980 Mar;3(2):150–164. doi: 10.1016/0147-619x(80)90106-7. [DOI] [PubMed] [Google Scholar]
- Covarrubias L., Cervantes L., Covarrubias A., Soberón X., Vichido I., Blanco A., Kupersztoch-Portnoy Y. M., Bolivar F. Construction and characterization of new cloning vehicles. V. Mobilization and coding properties of pBR322 and several deletion derivatives including pBR327 and pBR328. Gene. 1981 Jan-Feb;13(1):25–35. doi: 10.1016/0378-1119(81)90040-8. [DOI] [PubMed] [Google Scholar]
- Dendinger S. M., Brenchley J. E. Temperature-sensitive glutamate dehydrogenase mutants of Salmonella typhimurium. J Bacteriol. 1980 Dec;144(3):1043–1047. doi: 10.1128/jb.144.3.1043-1047.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendinger S. M., Patil L. G., Brenchley J. E. Salmonella typhimurium mutants with altered glutamate dehydrogenase and glutamate synthase activities. J Bacteriol. 1980 Jan;141(1):190–198. doi: 10.1128/jb.141.1.190-198.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Kinney D. M., Moat A. G. Pyridine nucleotide cycle of Salmonella typhimurium: isolation and characterization of pncA, pncB, and pncC mutants and utilization of exogenous nicotinamide adenine dinucleotide. J Bacteriol. 1979 Mar;137(3):1165–1175. doi: 10.1128/jb.137.3.1165-1175.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALPERN Y. S., UMBARGER H. E. Conversion of ammonia to amino groups in Escherichia coli. J Bacteriol. 1960 Sep;80:285–288. doi: 10.1128/jb.80.3.285-288.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Mecke D., Holzer H. Repression und inaktivierung von Glutaminsynthetase in Escherichia coli durch NH4+. Biochim Biophys Acta. 1966 Aug 10;122(2):341–351. [PubMed] [Google Scholar]
- Meers J. L., Tempest D. W., Brown C. M. 'Glutamine(amide):2-oxoglutarate amino transferase oxido-reductase (NADP); an enzyme involved in the synthesis of glutamate by some bacteria. J Gen Microbiol. 1970 Dec;64(2):187–194. doi: 10.1099/00221287-64-2-187. [DOI] [PubMed] [Google Scholar]
- Pahel G., Zelenetz A. D., Tyler B. M. gltB gene and regulation of nitrogen metabolism by glutamine synthetase in Escherichia coli. J Bacteriol. 1978 Jan;133(1):139–148. doi: 10.1128/jb.133.1.139-148.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzkin B., Roth J. Cluster of genes controlling proline degradation in Salmonella typhimurium. J Bacteriol. 1978 Feb;133(2):744–754. doi: 10.1128/jb.133.2.744-754.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld S. A., Brenchley J. E. Bacteriophage P1 as a vehicle for Mu mutagenesis of Salmonella typhimurium. J Bacteriol. 1980 Nov;144(2):848–851. doi: 10.1128/jb.144.2.848-851.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld S. A., Dendinger S. M., Murphy C. H., Brenchley J. E. Genetic characterization of the glutamate dehydrogenase gene (gdhA) of Salmonella typhimurium. J Bacteriol. 1982 May;150(2):795–803. doi: 10.1128/jb.150.2.795-803.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto N., Kotre A. M., Savageau M. A. Glutamate dehydrogenase from Escherichia coli: purification and properties. J Bacteriol. 1975 Nov;124(2):775–783. doi: 10.1128/jb.124.2.775-783.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Wharton R. P., Seltzer S., Kacinski B. M., Clarke N. D., Rupp W. D. Identification of the uvrA gene product. J Mol Biol. 1981 May 5;148(1):45–62. doi: 10.1016/0022-2836(81)90234-5. [DOI] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sánchez-Pescador R., Sanvicente E., Valle F., Bolivar F. Recombinant plasmids carrying the glutamate dehydrogenase structural gene from Escherichia coli K-12. Gene. 1982 Jan;17(1):1–8. doi: 10.1016/0378-1119(82)90095-6. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Synthesis of glutamate in Aerobacter aerogenes by a hitherto unknown route. Biochem J. 1970 Apr;117(2):405–407. doi: 10.1042/bj1170405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B. Regulation of the assimilation of nitrogen compounds. Annu Rev Biochem. 1978;47:1127–1162. doi: 10.1146/annurev.bi.47.070178.005403. [DOI] [PubMed] [Google Scholar]
- Varricchio F. Control of glutamate dehydrogenase synthesis in Escherichia coli. Biochim Biophys Acta. 1969 May 6;177(3):560–564. doi: 10.1016/0304-4165(69)90319-5. [DOI] [PubMed] [Google Scholar]
- Veronese F. M., Boccu E., Conventi L. Glutamate dehydrogenase from Escherichia coli: induction, purification and properties of the enzyme. Biochim Biophys Acta. 1975 Feb 19;377(2):217–228. doi: 10.1016/0005-2744(75)90304-6. [DOI] [PubMed] [Google Scholar]
- Windass J. D., Worsey M. J., Pioli E. M., Pioli D., Barth P. T., Atherton K. T., Dart E. C., Byrom D., Powell K., Senior P. J. Improved conversion of methanol to single-cell protein by Methylophilus methylotrophus. Nature. 1980 Oct 2;287(5781):396–401. doi: 10.1038/287396a0. [DOI] [PubMed] [Google Scholar]