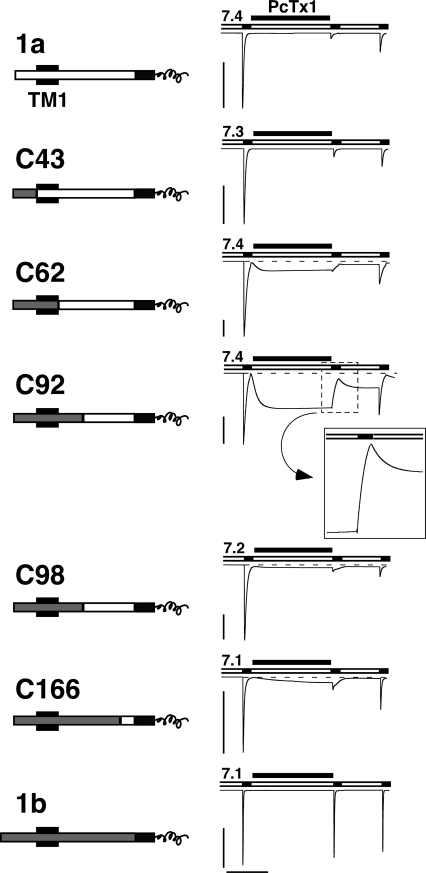
Figure 7.
PcTx1 inhibits chimeras between ASIC1a and 1b. Left, chimeras are schematically drawn. NH2-terminal sequences from ASIC1a are shown as open bars, those from ASIC1b as gray bars. The first transmembrane domain TM1 is indicated as a black box and the common COOH terminus is shown as a black bar; only its first part is shown. The chimeras did have a shorter NH2 terminus than ASIC1b; this shorter NH2 terminus corresponds to M3 in Bässler et al. (2001). However, since the missing part of the NH2 terminus of ASIC1b is supposed to be located in the cytoplasm and since chimera C43 interacted with PcTx1 like ASIC1a, this NH2-terminal part does not seem to have any strong influence on the interaction with PcTx1. Right, PcTx1 strongly inhibited the currents of ASIC1a and all chimeras, but not of ASIC1b. Conditioning pH was chosen according to the pH50 of steady-state desensitization of each channel. Acidic test pH was always pH 5.0. PcTx1 (50 nM) was applied in the conditioning period for 120 s. Note the opening of chimeras C61, C92, C98, and C166 by application of PcTx1. The transient decrease in current amplitude with C92, after washout of PcTx1 and application of pH 5.0, is enlarged. Scale bars correspond to 5 μA and 60 s, respectively. Peak current amplitudes (mean ± SD) were −11.6 ± 6.1 μA for ASIC1a (n = 7), −35.9 ± 18.2 μA for C43 (n = 6), −8.4 ± 9.9 μA for C61 (n = 6), −12.3 ± 4.5 μA for C91 (n = 5), −12.0 ± 4.0 μA for C98 (n = 3), −11.4 ± 3.1 μA for C166 (n = 5), and −11.1 ± 6.0 μA for ASIC1b (n = 9).