Abstract
Strongly inwardly rectifying potassium channels exhibit potent and steeply voltage-dependent block by intracellular polyamines. To locate the polyamine binding site, we have examined the effects of polyamine blockade on the rate of MTSEA modification of cysteine residues strategically substituted in the pore of a strongly rectifying Kir channel (Kir6.2[N160D]). Spermine only protected cysteines substituted at a deep location in the pore, between the “rectification controller” residue (N160D in Kir6.2, D172 in Kir2.1) and the selectivity filter, against MTSEA modification. In contrast, blockade with a longer synthetic polyamine (CGC-11179) also protected cysteines substituted at sites closer to the cytoplasmic entrance of the channel. Modification of a cysteine at the entrance to the inner cavity (169C) was unaffected by either spermine or CGC-11179, and spermine was clearly “locked” into the inner cavity (i.e., exhibited a dramatically slower exit rate) following modification of this residue. These data provide physical constraints on the spermine binding site, demonstrating that spermine stably binds at a deep site beyond the “rectification controller” residue, near the extracellular entrance to the channel.
INTRODUCTION
The term rectification is used to describe the property of certain ion channels to preferentially allow currents to flow in one direction (either into or out of the cell). Rectification is a critical feature of many functional groups of channels, including K+ channels and glutamate receptors. Within the structural family of inwardly rectifying K+ (Kir, KCNJ) channels, there is a spectrum of rectification properties that depends in large part on the presence of a negatively charged amino acid residue, often termed the “rectification controller” in the pore-lining M2 helix (Lu and MacKinnon, 1994; Wible et al., 1994; Nichols and Lopatin, 1997; Lu, 2004). Under physiological conditions, weakly rectifying channels (e.g., Kir6.2) allow considerable outward currents at depolarized potentials, whereas strongly rectifying channels (e.g., Kir2.1, Kir6.2[N160D]) are able to nearly completely prevent ion permeation in the outward direction (Nichols and Lopatin, 1997; Lu, 2004). Variability in the strength of inward rectification is related to differences in channel sensitivity to polyamines, with strongly rectifying channels exhibiting a potent and strongly voltage-dependent block by intracellular polyamines (Lopatin et al., 1994; Ficker et al., 1994; Fakler et al., 1995).
To block Kir channels, polyamines enter and occlude the central K+-selective pore of the channel. The affinity and voltage dependence of block varies with the identity of the blocking polyamine, spermine generally being the most potent and voltage-dependent blocker and shorter polyamines (e.g., spermidine, cadaverine, and putrescine) exhibiting weaker affinity and voltage dependence (Lopatin et al., 1995; Nichols and Lopatin, 1997; Pearson and Nichols, 1998; Guo and Lu, 2003; Guo et al., 2003). The steep voltage dependence of polyamine blockade likely arises in part from interactions of the blocking molecule with permeating ions, as movement of the blocker through the channel pore forces occupant permeant ions to traverse the membrane electric field (Spassova and Lu, 1998; Pearson and Nichols, 1998; Lu, 2004).
A general concept underlying interpretation of the voltage dependence of channel blockade is that it should correlate with the depth of the blocking site in the pore; entry of polyamines into a deep blocking site in Kir channels should displace more K+ ions (or traverse a larger fraction of the transmembrane field) than polyamines binding to a shallower site. And although it is well known that channel block by intracellular polyamines is the underlying mechanism of inward rectification, the details of this process, and particularly the specific physical location of polyamine binding, remain incompletely resolved (Lopatin et al., 1995; Guo et al., 2003; Kurata et al., 2004; John et al., 2004; Lu, 2004). Some studies have suggested a model of “shallow” spermine block of Kir channels, with spermine binding between the “rectification controller” residue and several rings of negatively charged residues located in the cytoplasmic domain of the channel (Guo and Lu, 2003; Guo et al., 2003). These authors have argued that binding of spermine at a relatively shallow site in the pore can result in a large voltage dependence of block by displacing a column of at least five K+ ions along the Kir pore (Lu, 2004; Shin and Lu, 2005). Others have proposed a “deep” model of spermine block, suggesting that spermine binds between the “rectification controller” residue and the selectivity filter (Chang et al., 2003; Kurata et al., 2004; John et al., 2004). In both the deep and shallow models, displacement of K+ ions by spermine is likely to account for a large fraction of the voltage dependence of block, but in the deep model, the blocker is proposed to reach a much deeper site in the pore, such that displacement of K+ ions from the selectivity filter is the logical source of the charge movement (Kurata et al., 2004; John et al., 2004). In this study, we address these contrasting models of polyamine blockade, using a novel variant of the “blocker protection” technique to determine the physical location of spermine binding in a Kir pore.
MATERIALS AND METHODS
KATP Channel Constructs and Expression in COSm6 Cells
General methods are described in detail in previous publications (Loussouarn et al., 2000). Point mutations were prepared by overlap extension at the junctions of relevant residues by sequential PCR as described. All cysteine mutations employed in these experiments (L157C, L164C, and M169C) were constructed on the Kir6.2[N160D][C166S] background construct, for several reasons. An earlier study (Loussouarn et al., 2000) demonstrated Cd2+ accessibility of residue C166; however, currents in the C166S channel are insensitive to Cd2+ or modification by MTSEA. In addition, the C166S mutant channel exhibits considerably less rundown than WT Kir6.2, which is advantageous during long inside-out patch clamp recordings (Trapp et al., 1998). The N160D mutation is included to confer steeply voltage-dependent, high affinity binding of spermine and other polyamines. Earlier studies of Kir6.2 have demonstrated that the N160D mutation in the Kir6.2 pore (equivalent to residue D172 in Kir2.1/IRK1 channels) confers a high affinity for polyamines, and an effective valence of spermine block (z∂ ∼ 4–5) essentially identical to that reported in Kir2.1 channels (Shyng et al., 1997; Guo et al., 2003; Kurata et al., 2004).
Patch-clamp Recording
COSm6 cells were transfected with pCMV6b-Kir6.2 (with mutations as described), pECE-SUR1, and pGreenLantern (Invitrogen), as previously described (Loussouarn et al., 2000; Phillips et al., 2003). Patch-clamp experiments were made at room temperature, in a chamber that allowed the solution bathing the exposed surface of the isolated patch to be changed rapidly. Data were normally filtered at 0.5–2 kHz; signals were digitized at 5 kHz and stored directly on computer hard drive using Clampex software (Axon Instruments, Inc.). The standard pipette (extracellular) and bath (cytoplasmic) solution used in these experiments had the following composition: 140 mM KCl, 1 mM EGTA, 1 mM K-EDTA, 4 mM K2HPO4, pH 7. (Guo and Lu, 2002). Spermine was purchased from FLUKA chemicals, putrescine was purchased from Sigma-Aldrich, and CGC-11179 was made available to us through CellGate Pharmaceuticals Inc. (Loussouarn et al., 2005). MTSEA (Toronto Research Chemicals) was dissolved in the standard recording solution on the day of experiments to make a 10 mM stock that was stored on ice. Further dilutions to 100 μM were prepared and used immediately for channel modification. Microsoft Solver was used to fit data by a least-squares algorithm.
RESULTS
Blocking Properties of Spermine and CGC-11179
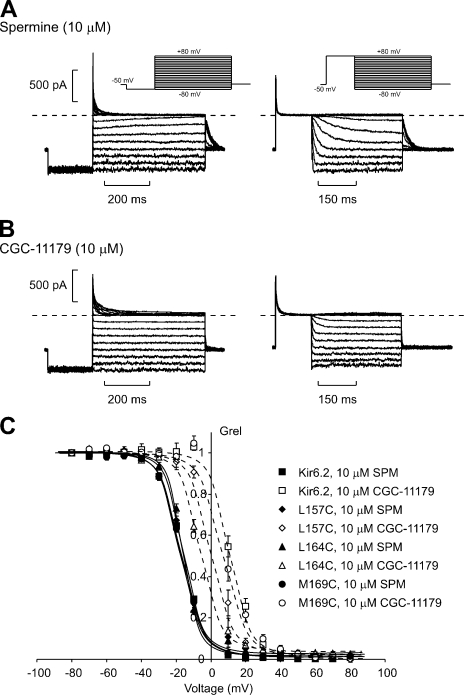
We have adopted the technique of “blocker protection” (Del Camino et al., 2000) to investigate the location at which various polyamines bind stably to Kir channels at depolarized voltages. We began by characterizing the blocking properties of spermine and a longer synthetic polyamine analogue (CGC-11179) in strongly rectifying Kir6.2[N160D][C166S] channels (Fig. 1). Spermine exhibits a steeply voltage-dependent block (zδ ∼ 4.5) that is not significantly altered by the introduction of cysteine residues at pore-lining residues 157, 164, or 169 in the M2 helix of Kir6.2 (Fig. 1 C). The synthetic polyamine CGC-11179 is a linear deca-amine, consisting of 10 amines separated by propyl linkers (for chemical structure, and size relative to spermine, see Fig. 8 or Loussouarn et al., 2005). In Kir6.2[N160D][C166S] and in cysteine-substituted channels (L157C, L164C, M169C), CGC-11179 exhibits slightly less potent block than spermine but with indistinguishable voltage dependence (Fig. 1 C).
Figure 1.
Blockade of Kir6.2[N160D] [C166S] channels by spermine and CGC-11179. (A and B) Spermine and CGC-11179 were applied at a concentration of 10 μM to the intracellular face of inside-out patches expressing Kir6.2[N160D][C166S] channels. Two protocols were used to quantify steady-state blocking parameters. In the left panels (blocking protocol), patches were held at −50 mV, pulsed for 200 ms to −80 mV, and then pulsed for 500 ms to voltages between 80 and +80 mV. In the right panels (unblocking protocol), patches were held at −50 mV, pulsed for 150 ms to +80 mV, and repolarized to voltages between +80 and −80 mV in 10-mV steps. (C) Steady-state currents at voltages between −80 and +80 mV were normalized to steady-state currents in the absence of blockers, for Kir6.2[N160D][C166S] and a number of cysteine-substituted channels (L157C, L164C, and M169C). Solid lines represent fitted Boltzmann functions for spermine block of each channel type, and dashed lines represent fitted Boltzmann functions for CGC-11179 block of each channel type.
Figure 8.
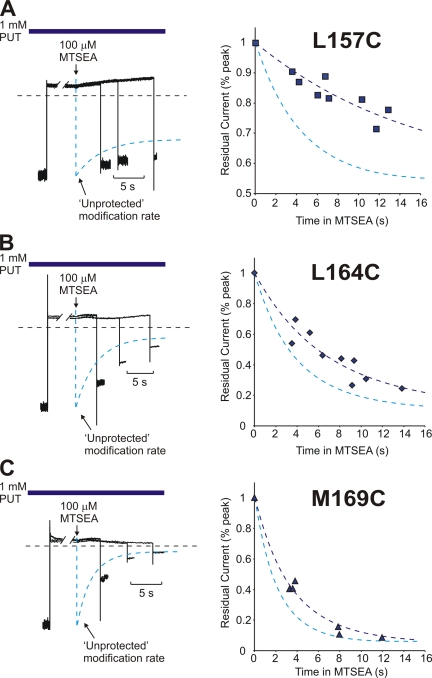
Protection of pore-lining cysteine residues by putrescine. Patches expressing (A) Kir6.2[N160D][C166S][L157C], (B) Kir6.2[N160D][C166S][L164C], or (C) Kir6.2[N160D][C166S][L169C] were blocked at +50 mV in 1 mM putrescine, exposed to 100 μM MTSEA for a variable interval (while continuously exposed to putrescine), and repolarized to −50 mV to determine the extent of MTSEA modification. Sample traces for each construct are presented in the lefthand panels, along with the unprotected MTSEA modification rates for comparison. Compiled data from multiple patches are presented in the righthand panels, and fit with a single exponential curve. Unprotected modification time courses are indicated by the dashed blue lines. At residue 157C, putrescine slowed the time constant of modification to 15.8 ± 1.3 s, from an unprotected time constant of 4.2 ± 0.7 s. At residue 164C in the presence of putrescine, the modification time constant was 7.1 ± 0.5 s, and the unprotected time constant was 3.9 ± 0.5 s. At residue 169C, putrescine slowed the modification time constant to 3.6 ± 0.3 s, from the unprotected time constant of 2.4 ± 0.3 s.
MTSEA Modification of the Kir6.2 Pore
Proximity or overlap of a bound polyamine with introduced cysteines in the Kir6.2 pore should interfere with the rate of cysteine modification by methanethiosulfonate reagents. To examine this, we first determined the rate of MTSEA modification of the various substituted cysteine residues (Fig. 2). Cysteine modification by MTSEA introduces a positively charged ethylamine adduct, and modification of cysteine residues substituted at pore-lining positions in Kir6.2 causes reduction of macroscopic current, reflecting a reduction of single channel current (Loussouarn et al., 2001; Phillips et al., 2003; Kurata et al., 2004). MTSEA application has no effect on ATP sensitivity (Phillips et al., 2003), and current is not rescued by PIP2, indicating that changes in open probability or channel rundown do not substantially contribute to the overall current reduction in MTSEA.
Figure 2.
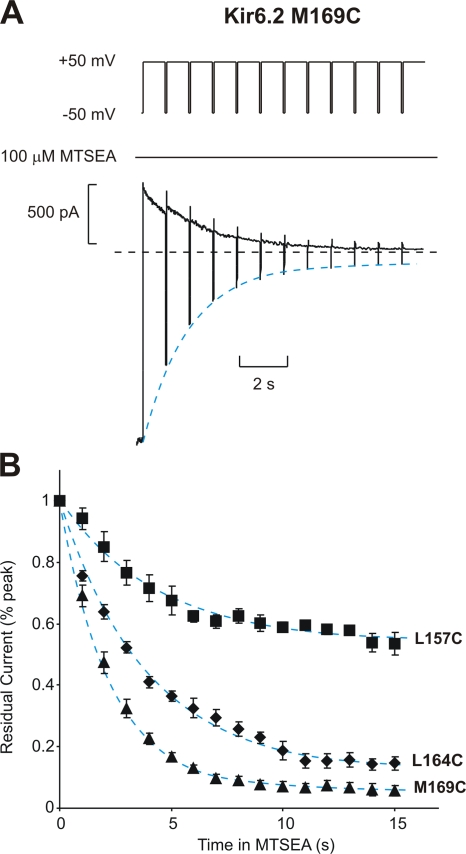
MTSEA modification of cysteine residues substituted in the Kir6.2 pore. (A) Sample data of modification of Kir6.2 [N160D][C166S][M169C] by 100 μM MTSEA. To characterize the rate of MTSEA modification at +50 mV, patches were held at +50 mV after application of 100 μM MTSEA to the intracellular side of the patch, and pulsed for 30 ms to −50 mV at 1-s intervals. (B) Mean data illustrating the modification rates of Kir6.2[N160D][C166S][L157C] (τ = 4.3 ± 0.7 s; n = 5), [L164C] (τ = 3.9 ± 0.3 s; n = 4), and [M169C] (τ = 2.3 ± 0.2 s; n = 4), channels by 100 μM MTSEA, measured as described in A. Dashed blue lines (here and throughout the text) represent monoexponential fits to the decay of residual currents by MTSEA modification, in the absence of any applied blocker.
The rate of MTSEA modification in various cysteine-substituted channels was determined as illustrated by the sample experiment in Fig. 2 A (the sample trace was collected from a patch expressing the M169C mutant channel). Immediately after MTSEA application, excised patches were pulsed to +50 mV with repeated brief repolarizations to −50 mV. This protocol was employed because MTSEA also blocks Kir6.2 in a voltage-dependent manner (Phillips et al., 2003), and brief repolarization to −50 mV is sufficient to relieve MTSEA block, allowing us to resolve the component of current reduction that is due to channel modification. The extent of current reduction depends upon the location of the substituted cysteine residue: modification of L164C or M169C channels reduces currents by 80–90%, whereas modification of L157C channels reduces currents by only ∼50%, as a result of differing effects on single channel currents (Fig. 2 B; Loussouarn et al., 2001; Phillips et al., 2003). The overall time course of the reduction of macroscopic currents in each cysteine mutant is well approximated by a monoexponential fit (dashed blue lines in Fig. 2, L157C τ = 4.2 ± 0.7 s, n = 5; L164C τ = 3.9 ± 0.5 s, n = 4; M169C τ = 2.4 ± 0.3 s, n = 4).
Stable Voltage-dependent Binding of Spermine and CGC-11179 in the Kir6.2 Pore
The unique property of polyamine block that makes the present study possible is the remarkably slow unbinding rate of long polyamines such as spermine and CGC-11179 at depolarized voltages. When control or cysteine-substituted channels are blocked with either spermine or CGC-11179 at +50 mV, and then the blockers rapidly removed from the bathing solution (with the membrane voltage held at +50 mV throughout), a very slow release of the blocker from the pore is apparent (Fig. 3). The recovery of peak current at +50 mV (τ > 1 min) reflects the very slow unbinding of the blockers at this voltage. There is a very rapid relief of block and restoration of current upon repolarization to −50 mV, confirming that neither polyamine application nor prolonged clamping of the membrane at +50 mV causes significant rundown of currents.
Figure 3.
Slow polyamine unbinding from the pore of mutant Kir6.2 channels. Patches expressing Kir6.2[N160D][C166S] were pulsed to +50 mV in (A) 10 μM spermine or (B) 10 μM CGC-11179. With the patch held continuously at +50 mV, the bathing solution was then switched to a polyamine-free solution to observe the time course of dissociation of polyamines from channels in the patch. A voltage step to −50 mV is sufficient to rapidly unblock either spermine or CGC-11179 from the channel and demonstrates that prolonged blockade and holding of the membrane potential at +50 mV does not result in significant channel rundown. The absence of significant blockade in a subsequent pulse to +50 mV demonstrates that most polyamine has diffused away from each patch. Similar experiments were performed on the cysteine-substituted mutants L157C, L164C, and M169C. In the lower panels, the exact details of voltage pulses and timing of solution changes have been omitted but are similar to those illustrated in the top row.
Blocker Protection in Kir6.2 by Spermine and CGC-11179
The extremely slow off-rate of spermine and CGC-11179 at +50 mV allowed a unique experimental design in our blocker protection assays. The approach was to “preblock” channels with either spermine or CGC-11179 and then apply MTSEA, with the expectation that blocker occupancy should interfere with MTSEA modification of cysteines at locations that overlap the binding site, without interference from free blocker in solution. This preblocking approach eliminates many complications that may arise if MTSEA and the blocker of interest are applied simultaneously, where kinetic differences in access to a binding site could potentially confuse the interpretation of data (see DISCUSSION). Modification of a cysteine that is protected by a polyamine cannot occur until the polyamine has unbound from the channel pore, so the remarkably slow off-rate of either polyamine is the limiting factor in the assay. Importantly, the modification rates of our cysteine-substituted channels in 100 μM MTSEA (Fig. 2 B) were substantially faster than the off-rates of spermine or CGC-11179 at +50 mV (Fig. 3, A and B), and so changes in the rate of MTSEA modification after preblocking with a polyamine could be readily resolved.
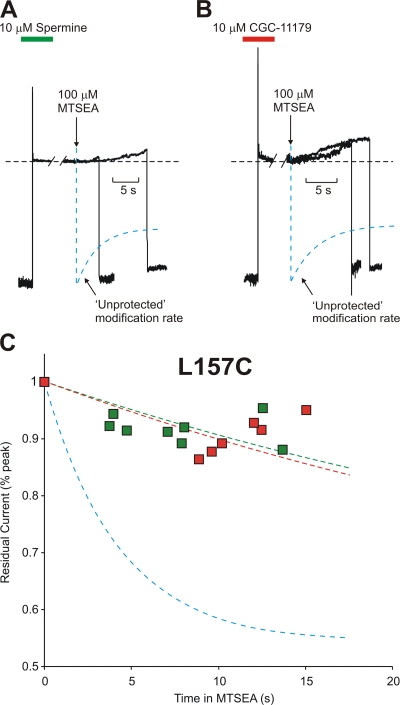
Sample traces from typical blocker protection experiments in the L157C channel are illustrated in Fig. 4 (A and B). From a holding potential of −50 mV, patches were pulsed to +50 mV in the presence of spermine (A) or CGC-11179 (B) to completely block channels. The bathing solution was then changed to a polyamine-free solution and, where indicated by the downward arrow, the patch was exposed to polyamine-free solution containing 100 μM MTSEA. Due to the slow off-rate of either polyamine (Fig. 3), channels remain blocked in these steps. After a variable interval, patches were repolarized to −50 mV and immediately removed from the MTSEA-containing solution. Repolarization to −50 mV resulted in release of any blocking spermine, allowing measurement of the residual current after MTSEA exposure. Superimposed on the raw data is the monoexponential fit (dashed blue line) of the MTSEA modification rate in “unprotected” (i.e., unblocked) L157C channels (from Fig. 2). A considerably larger residual current remained when channels were modified after preblocking with either spermine (Fig. 4 A) or CGC-11179 (Fig. 4 B).
Figure 4.
Protection of residue 157C by spermine or CGC-11179 occupancy of the Kir6.2 pore. Patches expressing Kir6.2[N160D][C166S][L157C] were preblocked by voltage steps to +50 mV in either (A) 10 μM spermine or (B) CGC-11179. While held continuously at +50 mV, patches were moved into a polyamine-free solution and, where indicated by the downward arrow, exposed to a polyamine-free solution containing 100 μM MTSEA. After variable intervals in 100 μM MTSEA, patches were repolarized to −50 mV (to assess the extent of MTSEA modification) and immediately removed from the MTSEA-containing solution. The unprotected modification rate (dashed blue line) represents the rate of MTSEA modification of L157C channels in polyamine-free conditions (from Fig. 2 B), and is superimposed on the raw data for comparison. (C) Modification of channels preblocked with either CGC-11179 (red symbols, τ = 52 ± 11 s) or spermine (green symbols, τ = 44 ± 7 s) was measured in multiple patches after varying intervals in 100 μM MTSEA to determine the time course of modification when the pore is occupied by either polyamine. The unprotected modification time course of L157C is indicated by the blue line (τ = 4.2 ± 0.7 s). Preblocking with either spermine or CGC-11179 strongly protects against MTSEA modification at residue 157C.
Experiments were performed on multiple patches with varied intervals in 100 μM MTSEA to determine the time course of MTSEA modification in channels preblocked with either spermine or CGC-11179 (Fig. 4 C). Importantly, we have previously shown that MTSEA modification of certain residues in the Kir6.2 pore can significantly affect the kinetics and affinity of spermine block (Kurata et al., 2004). Therefore, each patch can only be used once in these experiments, as any modification occurring in the first “run” can affect spermine occupancy and the apparent rate of modification in subsequent runs. Thus, each data point in Fig. 4 C is from a different patch, and not from cumulative MTSEA treatment of a single patch. Overall, the rate of MTSEA modification of L157C channels was slowed ∼10-fold after preblocking with either CGC-11179 (Fig. 4 C, red symbols; τ = 52 ± 11 s; unprotected τ = 4.2 ± 0.7 s) or spermine (Fig. 4 C, green symbols; τ = 44 ± 7 s). Thus, residue 157C (located between the “rectification controller” residue and the selectivity filter) is strongly protected against MTSEA modification by occupancy of the pore with either spermine or CGC-11179.
Accessibility of Residue 164C in Spermine-blocked Channels
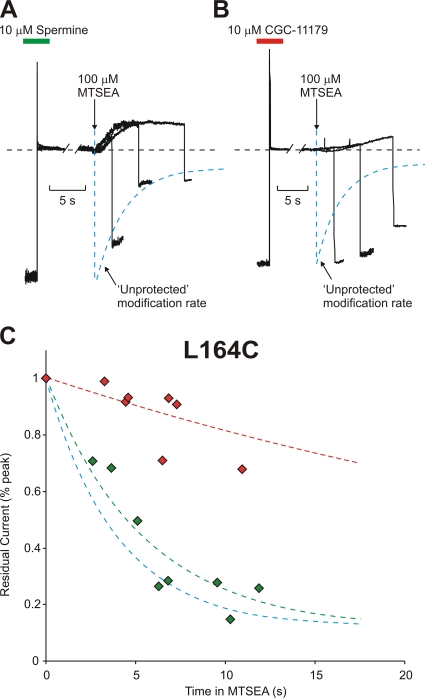
Similar protection experiments were conducted at several other sites in the Kir6.2 pore and, importantly, the profile of protection changes significantly with position. Most strikingly, at a slightly more shallow position in the inner cavity (L164C, one turn of the M2 helix below the rectification controller residue, toward the intracellular entrance of the channel), preblocking with spermine offers essentially no protection against MTSEA modification (Fig. 5, A and C; spermine τ = 5.4 ± 0.5 s; unprotected τ = 3.9 ± 0.5 s). In contrast, preblocking with the long polyamine analogue CGC-11179 still strongly protects against MTSEA modification at this position (Fig. 5, B and C; CGC-11179 τ = 35 ± 8 s). These results indicate that bound CGC-11179 overlaps with residue 164C, and occludes modification by MTSEA, while the shorter spermine fails to interfere with access of MTSEA to residue 164C.
Figure 5.
Residue 164C is differentially protected by spermine or CGC-11179 occupancy of the Kir6.2 pore. Patches expressing Kir6.2[N160D][C166S][L164C] channels were preblocked by voltage steps to +50 mV in either (A) 10 μM spermine or (B) CGC-11179. As described in Fig. 4, patches were moved into a polyamine-free solution and exposed to a solution containing 100 μM MTSEA where indicated by the downward arrow. After variable intervals in 100 μM MTSEA, patches were repolarized to −50 mV and immediately removed from the MTSEA-containing solution. (C) Modification of channels preblocked with either CGC-11179 (red symbols, τ = 35 ± 8 s) or spermine (green symbols, τ = 5.4 ± 0.5 s) in multiple patches. The unprotected modification of L164C is indicated by the blue line (τ = 3.9 ± 0.5 s). Preblocking with spermine does not prevent MTSEA modification of 164C, while CGC-11179 protects strongly at this position.
We have previously demonstrated that the introduction of positive charges at position L164C dramatically reduces the channel affinity for spermine, with a pronounced acceleration of spermine off-rate (Kurata et al., 2004). This is most likely due to the close proximity of residues 164 and 160, such that introduction of positive charges at residue 164 counteracts the negatively charged rectification controller at residue N160D. An interesting consequence of this property of MTSEA-modified L164C channels becomes apparent in the spermine preblocking experiments (Fig. 5 A). Since spermine binding is essentially abolished in MTSEA-modified L164C channels, one would expect that MTSEA modification of a preblocked 164C channel would lead to exit of the blocking spermine ion shortly thereafter. This is indeed reflected in the experimental data; the application of MTSEA to L164C channels preblocked with spermine causes rapid relief of spermine block (Fig. 5 A), considerably faster than the intrinsic rate of spermine unbinding in the absence of MTSEA (compare with L164C in Fig. 3 A). In contrast, accelerated unblock is not apparent when MTSEA is applied to 164C channels preblocked with CGC-11179 (Fig. 5 B). Thus, 164C is protected in CGC-11179-blocked channels and not accessible to MTSEA. In spermine-blocked channels, the 164C residue is accessible to MTSEA, with modification leading to a decrease in spermine affinity and rapid exit of spermine from the pore. The result is channel unblock (with kinetics similar to the rate of 164C modification) to a current level corresponding to the fully MTSEA modified state of the channels (Fig. 5 A).
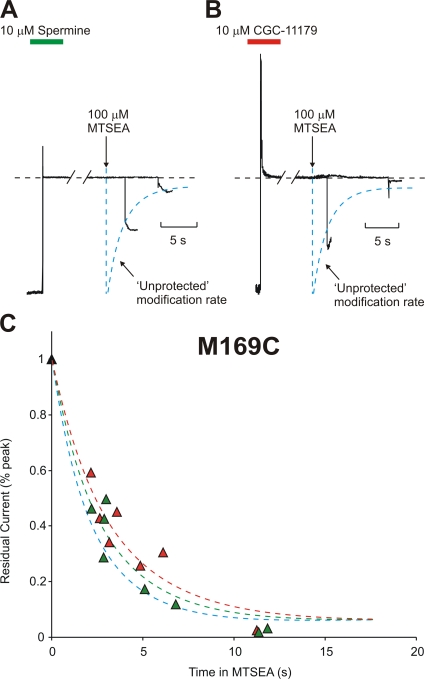
Blocker Protection Is Absent at Residue 169C
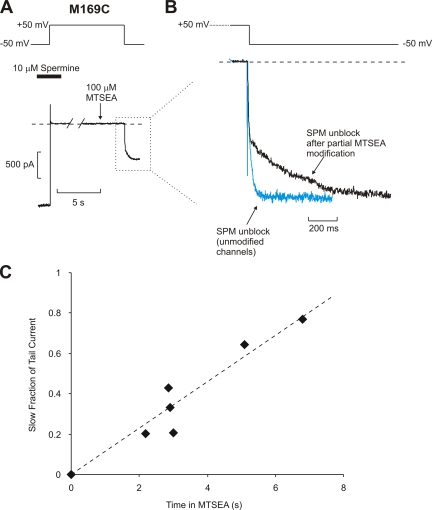
The profile of protection is different again at residue 169, which is located at the cytoplasmic end of the inner cavity. Preblocking with either spermine or CGC-11179 fails to substantially alter modification of M169C by MTSEA (Fig. 6, spermine τ = 2.7 ± 0.2 s; CGC-11179 τ = 3.3 ± 0.3 s; unprotected τ = 2.4 ± 0.3 s). We previously demonstrated that after MTSEA modification of residue 169C, entry and exit of spermine from the inner cavity is considerably slowed (Kurata et al., 2004); the preblocking protection experiments presented here reinforce this point. As shown in Fig. 6 C, the rate of M169C modification is not altered when channels have been preblocked. However, after the MTSEA modification step in preblocked channels, there is an obvious bi-exponential time course of current recovery upon repolarization, due to the appearance of a slow activation component (τ = 360 ± 40 ms; n = 6) that reflects the slow unbinding of spermine from MTSEA-modified channels at −50 mV. This phenomenon is apparent in the sample traces shown in Fig. 6 A, and has been expanded in Fig. 7 (A and B). To demonstrate this point further, we have compiled data from several 169C patches exposed to MTSEA for varying durations (Fig. 7 C). As the time of exposure is prolonged, the relative weight of the slow component is increased, as expected if the slow component is related to trapping of spermine by modification of the 169C residue. Thus, MTSEA modification of 169C is unaltered by the presence of spermine, and modification actually traps the blocker in the inner cavity. The dramatic slowing of the spermine off-rate also illustrates that preblocked spermine molecules can remain within the Kir6.2 pore during and after the modification step at residue 169. There is no obvious slowing of CGC-11179 unblock after modification. However, the off-rate of CGC-11179 is considerably faster than that of spermine at −50 mV (Fig. 1, A and B), leaving us unable to resolve with certainty whether MTSEA modification of residue 169C can also trap the CGC-11179 compound in the pore.
Figure 6.
Residue 169C is not protected by spermine or CGC-11179 occupancy of the Kir6.2 pore. Patches expressing Kir6.2 [N160D][C166S][M169C] were preblocked by voltage steps to +50 mV in either (A) 10 μM spermine or (B) CGC-11179 and exposed to a polyamine-free solution containing 100 μM MTSEA, as described in Figs. 4 and 5. (C) Modification of channels preblocked with either CGC-11179 (red symbols, τ = 3.3 ± 0.3 s) or spermine (green symbols, τ = 2.7 ± 0.2 s) in multiple patches. The time course of unprotected modification of M169C is indicated by the blue line (τ = 2.4 ± 0.3 s). Pore occupancy by either polyamine does not significantly alter the rate of cysteine modification at 169C.
Figure 7.
MTSEA modification of M169C traps spermine in the Kir6.2 pore. (A) Sample data of a blocker protection experiment of Kir6.2[N160D][C166S][M169C] channels preblocked with spermine, collected as described in Figs. 4–6 . (B) Expanded data illustrating the tail currents observed in A upon repolarization to −50 mV (black trace). The blue trace, included for comparison, illustrates the rate of spermine unblock from unmodified M169C channels. The slow unblocking time course in modified M169C channels demonstrates that spermine remains bound in the pore during the modification step, and is effectively trapped by the introduction of positive charges at residue 169.
Protection Effects of Putrescine in the Kir Pore
To characterize the localization of polyamines in the pore in more detail, we also determined the protection profile of putrescine in cysteine-substituted channels. These experiments require a slightly different experimental design than described earlier for spermine and CGC-11179, because putrescine does not exhibit the remarkably slow off-rate at +50 mV that is characteristic of the longer polyamines. Therefore, 1 mM putrescine was maintained in the bathing solution throughout the entire protocol, ensuring that a significant fraction of channels were blocked during the application of MTSEA. Apart from this detail, the design was identical to the experiments described in Figs. 4–6 . Patches expressing L157C (Fig. 8 A), L164C (Fig. 8 B), or M169C (Fig. 8 C) mutants were preblocked in putrescine at +50 mV, exposed to 100 μM MTSEA for a variable duration (with persistent exposure to putrescine), and repolarized to −50 mV to determine the extent of current reduction due to MTSEA exposure. Data from multiple patches of each mutant are compiled in Fig. 8 (righthand panels) together with the unprotected modification rates (from Fig. 2) at each position. At position L157C, putrescine occupancy resulted in significant protection (τ = 15.8 ± 1.3 s; unprotected τ = 4.2 ± 0.7 s), although the effects were more modest than the protection of this site by spermine and CGC-11179 (Fig. 4). The protective effects of putrescine are smaller at both 164C (Fig. 8 B; τ = 7.1 ± 0.5 s; unprotected τ = 3.9 ± 0.5 s) and 169C (Fig. 8 C; τ = 3.6 ± 0.3 s; unprotected τ = 2.4 ± 0.3 s). While the protective effects of putrescine appear to be more diffuse than for spermine or CGC-11179, which may reflect the technical limitations of the protocol (see DISCUSSION), residue 157C is clearly the most strongly protected of the three residues examined (Fig. 8).
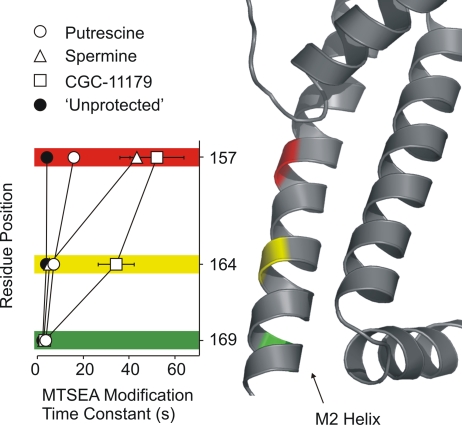
The protection profile of each blocker is summarized in Fig. 9, where the mean unprotected and protected time constants of MTSEA modification are plotted at each residue examined. The plot is lined up with a depiction of the KirBac1.1 M2 helix, with colors highlighting the equivalent residues examined in the present study (Kuo et al., 2003). Residue L157C is strongly protected by spermine and CGC-11157, and less so by putrescine. Modification of L164C is substantially slowed only in the presence of CGC-11179, and no blockers protected L169C channels from modification.
Figure 9.
Spatial orientation of substituted cysteines in the Kir pore. Summary of the time constants of MTSEA modification (mean ± SEM) at residues 157C, 164C, and 169C, in the presence of putrescine, spermine, CGC-11179, or no blocker (unprotected), as measured in Figs. 2–8 . A representation of the M2 helix, based on the X-ray structure of KirBac1.1, is aligned with the plot to illustrate the relative positions of the substituted cysteine residues in the inner cavity.
DISCUSSION
Molecular Basis of Polyamine Block
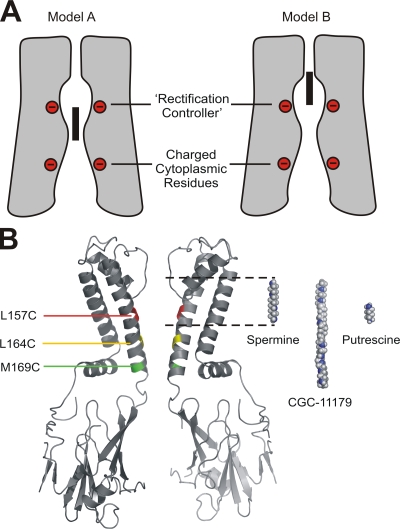
Steeply voltage-dependent block by polyamines accounts for the unique rectification properties of strong inwardly rectifying potassium channels (Lopatin et al., 1994; Ficker et al., 1994; Fakler et al., 1995; Guo and Lu, 2002). However, the molecular details underlying this process have remained controversial, particularly with regard to the physical location of spermine binding (Guo and Lu, 2003; Kurata et al., 2004; John et al., 2004). Crystal structures have revealed that the pores of inwardly rectifying potassium channels are considerably longer than an individual spermine molecule and are lined by multiple rings of negative charges (Kuo et al., 2003). This has led to one proposed model in which spermine and other polyamines are bound stably between the negatively charged rectification controller residue in the inner cavity (D172 in Kir2.1, equivalent to N160D in Kir6.2 examined in the present study) and multiple negatively charged residues in the cytoplasmic domain of the channel (Fig. 10, Model A; Nishida and MacKinnon, 2002; Guo and Lu, 2003; Guo et al., 2003; Pegan et al., 2005). With relatively shallow spermine binding in the Kir pore, the voltage dependence of polyamine block must then arise entirely from the obligate displacement of a column of K+ ions as a polyamine molecule approaches its binding site (Lu, 2004; Shin and Lu, 2005). An alternative model is a deeper binding site for spermine in the inner cavity, between the rectification controller residue and the selectivity filter (Chang et al., 2003; Dibb et al., 2003; John et al., 2004; Kurata et al., 2004), with the head of spermine lying near or within the selectivity filter (Fig. 10 A, Model B). In this case, charge movement can be the result both of significant polyamine movement through the membrane field and displacement of K+ ions from the inner cavity and the selectivity filter.
Figure 10.
The polyamine binding site in the Kir channel pore. (A) Cartoons to illustrate contrasting models of shallow (Model A) versus deep spermine binding (Model B). Red circles indicate rings of negative charges in the cytoplasmic domain (bottom circles) and the inner cavity (top circles) of strongly rectifying Kir channels. The black rectangle represents a spermine molecule in the Kir pore. (B) Using the KirBac1.1 crystal structure as a template, we have mapped the examined residues and colored them to reflect the protection profile by spermine and CGC-11179. Residue 157 (red) is protected against MTSEA modification by both spermine and CGC-11179 (see Fig. 4). Residue 164 (yellow) is protected by CGC-11179 but not by spermine (see Fig. 5). Residue 169 (green) is not protected by either polyamine (see Fig. 6). We have also aligned spermine, CGC-11179, and putrescine molecules with binding locations indicated by the observed protection profile. The head of spermine and CGC-11179 are placed near the entrance to the selectivity filter. The tail of spermine extends to the approximate location of the rectification controller residue (N160D in Kir6.2), while the considerably longer CGC-11179 molecule extends to the inner cavity entrance. Putrescine is located near the rectification controller residue.
Several studies have now employed thermodynamic mutant cycle analysis to probe the location of spermine block. Varying conclusions have been drawn and support has been argued for each of the models above (Guo and Lu, 2003; Guo et al., 2003; Kurata et al., 2004). In all instances, the analysis has been hampered by the drawback that ΔΔG values have been derived from changes in apparent “overall” Kd values, and thus interpreted in the context of a single barrier binding equilibrium. However, it has long been known that at least two sequential equilibria are required to adequately describe the kinetic and steady-state properties of spermine block in Kir2.1, with a peripheral, only weakly voltage-dependent binding and a deeper voltage-dependent site responsible for steep rectification (Lopatin et al., 1995; Shin and Lu, 2005). In such a sequential model, mutations that alter an early equilibrium, but leave the deep spermine binding site unchanged, can affect the apparent Kd (see Eq. 1a in Shin and Lu, 2005). If interpreted in terms of a single barrier model, this will incorrectly imply disruption of the deep site responsible for steep voltage-dependent rectification. Given these significant potential pitfalls for interpretation of mutant cycle analyses, a blocker protection study potentially provides a far more direct approach for identifying the physical location of polyamine binding sites.
Blocker Protection Profile of Spermine and CGC-11179
The blocker protection properties we have described for spermine and CGC-11179 seem to exclude stable binding at more shallow sites in the Kir pore and clearly support a model in which the blockers bind at a deep site. In the residues examined here, spermine protected only residue 157C (between the rectification controller and selectivity filter) from modification. Modification of residues at more shallow sites in the pore (164C and 169C) was unaltered by the presence of preblocked spermine. Protection of residues at the more shallow 164C location required preblocking with the much longer synthetic polyamine CGC-11179, while at the most shallow location examined (169C), even CGC-11179 did not hinder modification by MTSEA. The protection profile of CGC-11179, when compared with the length of this compound (see Fig. 10, and later DISCUSSION), suggests that the head of this compound binds at a very deep site in the transmembrane region of the channel. The protection profile for spermine (together with its indistinguishable effective valence relative to CGC-11179, Fig. 1 C) suggests a similarly deep binding site.
Many blocker protection studies have applied MTS reagents in the continuous presence of a blocker and have repetitively relieved block by voltage pulses to observe the extent of modification (Del Camino et al., 2000; Chang et al., 2003). In Kir channels, an important potential ambiguity arising from this approach results from modification reducing spermine affinity (Kurata et al., 2004). A conceivable situation is one in which rapid MTSEA entry into the inner cavity could precede blockade by spermine, allowing modification to take place before spermine reaches its binding site. If periodic voltage pulses are used to assess the extent of modification, this problem could be compounded with each repetitive pulse. At a location such as residue 157C, MTSEA modification does significantly reduce the potency and dwell time of spermine block (Kurata et al., 2004), and would thus reduce the ability of spermine to protect this site. Preblocking channels with either spermine or CGC-11179, and avoiding the use of repetitive voltage pulses, avoids the possibility that kinetic differences in access rates between spermine/CGC-11179 and MTSEA could mask or attenuate protection by a polyamine occupying the pore. The different protocol may account for some discrepancies in the results of our study compared with an earlier study in Kir2.1 (Chang et al., 2003), particularly the apparent absence of significant protection of Kir2.1 residue 169C (equivalent to 157C in our study) by spermine. It is reassuring, however, that both our study and a previous study (Chang et al., 2003) reported strong protection at deep sites in the Kir pore. While Chang et al. (2003) also reported some protection of Kir2.1 residue 176C (equivalent to 164C in our study), this effect was very modest compared with deeper sites in the pore, indicating that the model of deep spermine binding extends to the physiologically important strongly rectifying channel Kir2.1.
A second consideration in the interpretation of these data is the volume or capacity of the inner cavity. The data are significantly different from what one would predict based on a model of shallow polyamine binding (Guo and Lu, 2003; Guo et al., 2003), and before dismissing it, we have considered the possibility that MTSEA could bypass spermine and modify residues that in fact overlap or lie beyond the spermine binding site. It has been suggested, for example, that the relatively weak voltage dependence of block by divalent cations such as Ba2+ and Mg2+ might involve them bypassing K+ ions in the pore (and hence not requiring movement of K+ ions through the field), to reach a blocking site that is considerably deeper than has been proposed for spermine (Jiang and MacKinnon, 2000; Lu, 2004). While space-filling considerations suggest that this is improbable in the present case (given the substantially larger sizes of spermine and MTSEA relative to Ba2+ or K+), this issue provided a major impetus for examination of CGC-11179 (Loussouarn et al., 2005). Importantly, we observed a clear extension of the protected region of the inner cavity when occupied by CGC-11179 vs. spermine (Figs. 5 and 9), arguing against the possibility that MTS reagents are somehow bypassing the blocking polyamine to access substituted cysteine residues.
Models of Polyamine Binding in the Kir Pore
In Figs. 9 and 10, we have mapped the residues examined in the present study onto equivalent positions in the published crystal structure of the KirBac1.1 channel (Kuo et al., 2003). The mapped residues have been color coded, based on their protection profile. Residue L157 (red, equivalent to M135 in KirBac) is protected by both spermine and CGC-11179. Residue L164 (yellow, equivalent to T142 in KirBac) is protected by CGC-11179, but not spermine. The shallowest residue examined (M169, green, equivalent to A147 in KirBac) is not protected by either spermine or CGC-11179. Although the experimental design differed somewhat, and the protection effects were considerably smaller than for spermine and CGC-11179, putrescine only protected residue 157C, with little or no protection of residues 164C and 169C (Figs. 8 and 9). Adjacent to the full channel structure in Fig. 10, we have shown structures of fully extended spermine, CGC-11179, and putrescine, positioned in locations that are consistent with our data. The leading amines of both spermine and CGC-11179 are placed at a similar location, reflecting their indistinguishable effective valences (Fig. 1). Spermine is located between the rectification controller residue (160D) and the selectivity filter, accounting for its inability to protect against MTSEA modification of residues 164 and 169. The considerably longer CGC-11179 extends further toward the cytoplasmic vestibule of the channel, where it is able to protect against MTSEA modification of residue 164. Importantly, even with its head placed in the entrance to the selectivity filter, a fully extended CGC-11179 molecule would still extend slightly beyond residue 169, suggesting that this extremely long and flexible polyamine may not remain in its fully extended conformation in the inner cavity. Although the boundaries of the protection effects of putrescine are not as clear as those observed for spermine and CGC-11179, partial protection of only 157C, located above the rectification controller residue, is entirely consistent with the proposal that it binds between rectification controller and the entrance of the selectivity filter (Fig. 10).
Previous characterization of Kir2.1 channels has demonstrated that the effective valence of block by diamines and polyamines increases up to a maximum of ∼5 at an alkyl chain length of eight or nine (Pearson and Nichols, 1998; Guo et al., 2003). The shallow binding model proposed by Guo et al. located the trailing amine of diamines or of spermine between the rectification controller and the negatively charged residues in the cytoplasmic domain of the channel (Fig. 10, Model A). With longer polyamines/diamines, the leading amine was proposed to reach deeper into the pore toward the rectification controller residue, resulting in the displacement of more K+ ions, with a larger effective valence in consequence (Guo et al., 2003). One important potential problem with this model is that it seems to imply multiple K+ ions around the entrance to or in the inner cavity. That is, if the trailing amines of diamines/polyamines bind at essentially a fixed location, the difference in the position of the leading amines of putrescine and spermine, for example, would be only 10–12 Å. In the model of Lu and colleagues, this difference would need to account for a difference in valence of ∼3, suggesting the displacement of three additional K+ ions through the membrane field. However, there is little or no evidence to suggest such close spacing of K+ ion binding sites at this shallow location in the pore.
The blocker protection data in the present study seems to rule out stable binding of polyamines at a shallow location in the pore, as residue 169C is not protected by any of the polyamines examined (Figs. 6 and 8). The protection profile is far more consistent with the alternative model of deep spermine binding, in which the trailing amine of diamines or polyamines binds near the rectification controller, with the leading amine approaching or entering the selectivity (Fig. 10 B). This model essentially places spermine and other diamine blockers between the rectification controller and the selectivity filter, and the displacement of additional (closely spaced) K+ ions from the selectivity filter by the longest polyamines can then logically account for their larger effective valence (for various descriptions of this model see Chang et al., 2003; Dibb et al., 2003; Phillips and Nichols, 2003; John et al., 2004; Kurata et al., 2004). Importantly, the displacement of ions from binding sites identified in KcsA (one K+ ion in the inner cavity, and two K+ ions in the selectivity filter) could generate a maximal effective valence of 3, clearly insufficient to make up the large valence associated with spermine block. To account for this discrepancy, one possibility is displacement of K+ ions from additional binding sites at more shallow locations in the pore. It has been suggested that there may be one or more K+ binding sites in the cytoplasmic domain of Kir2.1 (Nishida and MacKinnon, 2002; Shin et al., 2005), and the crystal structure of KvAP appears to contain two K+ ions in the inner cavity (rather than a single cavity ion, as in KcsA) (Jiang et al., 2003). A second possibility is that one or more amines of a blocking spermine ion traverse a segment of the transmembrane field, and thus directly contribute to the valence of block. This could potentially arise by partial entry of spermine into the selectivity filter (see below), or if the distribution of the transmembrane field in Kir channels differed from that predicted in MthK (where the field drops almost entirely across the selectivity filter) and extended partially into the inner cavity.
Selectivity Filter Entry of Polyamines
Although the present study unambiguously indicates a spermine binding site deep in the inner cavity, an issue that remains difficult to resolve is whether it is plausible that spermine block involves entry into the selectivity filter. This remains an important question in understanding the mechanism underlying strong voltage dependence of polyamine block. One recent study demonstrated that steeply voltage-dependent block is maintained in a polyamine analogue with expanded head groups (decane-bis-trimethylammonium). This study concluded that block occurs in or below the inner cavity (Shin and Lu, 2005), but this hinges on the assertion that the bis-trimethylammonium head cannot enter the filter. Other studies have presented evidence consistent with slow permeation of spermine and other polyamines through Kir channels, indicating that barriers for spermine entry into (and even permeation through) the selectivity filter are not insurmountable (Guo and Lu, 2000a,b; Dibb et al., 2003; Makary et al., 2005).
We have also suspected spermine binding near or within the selectivity filter based on comparisons with the well-characterized properties of quaternary ammonium ions. Although the physical location of the intracellular binding site for TEA and other quaternary ammonium ions in Kir channels is not completely understood, all structural evidence (Zhou et al., 2001; Lenaeus et al., 2005) and blocker protection studies (Del Camino et al., 2000) suggest that these blockers occupy the cavity ion or dehydration transition site in the inner cavity of several other model K+ channels (i.e., Shaker and KcsA). In Kir channels, the effective valence of TEA blockade is normally <2 (Guo and Lu, 2001), while that of spermine is considerably larger. The possibility remains that the quaternary ammonium blocking site in Kirs differs significantly from other K+ channels (Shin et al., 2005). However, these observations collectively suggest that TEA may actually reach the cavity ion or dehydration transition site in Kir channels to achieve its effective valence. More careful determination of the quaternary ammonium binding site in Kir channels, by either structural studies or blocker protection studies, will likely provide significant new insights into this issue. The deep binding model can explain the even larger effective valence of spermine by entry into the selectivity filter and displacement of filter K+ ions (Kurata et al., 2004), a proposal that is entirely consistent with the results of our present study. Also consistent with this suggestion, and supporting the hypothesis that the selectivity filter comprises a final barrier to the exit of spermine to the extracellular solution, is the finding that disruption of the selectivity filter by mutagenesis of ion pairs in the P-loop of Kir2.1 and Kir3.1/3.4 can abolish spermine block, and instead allow spermine permeation (Yang et al., 1997; Dibb et al., 2003; Makary et al., 2005).
Conclusion
Pore occupancy by spermine can inhibit MTSEA modification of cysteine residues substituted at pore-lining positions in the pore of Kir6.2[N160D] channels. The pattern of protection is extended to more shallow pore-lining residues when channels are blocked with the extended polyamine analogue CGC-11179. The data unambiguously support a model of strong inward rectification in which spermine stably binds with its trailing amine near the rectification controller residue (D172 in Kir2.1, N160D in Kir6.2) and its leading amine located near or within the selectivity filter.
Acknowledgments
We are grateful to Gildas Loussouarn for many useful discussions early in this project.
This work was supported by National Institutes of Health grant HL54171 (to C.G. Nichols). H.T. Kurata is supported by a Canadian Institutes of Health Research Fellowship.
Lawrence G. Palmer served as editor.
Abbreviation used in this paper: MTSEA, 2-aminoethyl methanethiosulfonate.
References
- Chang, H.K., S.H. Yeh, and R.C. Shieh. 2003. The effects of spermine on the accessibility of residues in the M2 segment of Kir2.1 channels expressed in Xenopus oocytes. J. Physiol. 553:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Camino, D., M. Holmgren, Y. Liu, and G. Yellen. 2000. Blocker protection in the pore of a voltage-gated K+ channel and its structural implications. Nature. 403:321–325. [DOI] [PubMed] [Google Scholar]
- Dibb, K.M., T. Rose, S.Y. Makary, T.W. Claydon, D. Enkvetchakul, R. Leach, C.G. Nichols, and M.R. Boyett. 2003. Molecular basis of ion selectivity, block, and rectification of the inward rectifier Kir3.1/Kir3.4 K+ channel. J. Biol. Chem. 278:49537–49548. [DOI] [PubMed] [Google Scholar]
- Fakler, B., U. Brandle, E. Glowatzki, S. Weidemann, H.P. Zenner, and J.P. Ruppersberg. 1995. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 80:149–154. [DOI] [PubMed] [Google Scholar]
- Ficker, E., M. Taglialatela, B.A. Wible, C.M. Henley, and A.M. Brown. 1994. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 266:1068–1072. [DOI] [PubMed] [Google Scholar]
- Guo, D., and Z. Lu. 2000. a. Mechanism of cGMP-gated channel block by intracellular polyamines. J. Gen. Physiol. 115:783–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, D., and Z. Lu. 2000. b. Mechanism of IRK1 channel block by intracellular polyamines. J. Gen. Physiol. 115:799–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, D., and Z. Lu. 2001. Kinetics of inward-rectifier K+ channel block by quaternary alkylammonium ions. dimension and properties of the inner pore. J. Gen. Physiol. 117:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, D., and Z. Lu. 2002. IRK1 inward rectifier K+ channels exhibit no intrinsic rectification. J. Gen. Physiol. 120:539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, D., and Z. Lu. 2003. Interaction mechanisms between polyamines and IRK1 inward rectifier K+ channels. J. Gen. Physiol. 122:485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, D., Y. Ramu, A.M. Klem, and Z. Lu. 2003. Mechanism of rectifi- cation in inward-rectifier K+ channels. J. Gen. Physiol. 121:261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, V. Ruta, M. Cadene, B.T. Chait, and R. MacKinnon. 2003. X-ray structure of a voltage-dependent K+ channel. Nature. 423:33–41. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., and R. MacKinnon. 2000. The barium site in a potassium channel by x-ray crystallography. J. Gen. Physiol. 115:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, S.A., L.H. Xie, and J.N. Weiss. 2004. Mechanism of inward rectification in Kir channels. J. Gen. Physiol. 123:623–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, A., J.M. Gulbis, J.F. Antcliff, T. Rahman, E.D. Lowe, J. Zimmer, J. Cuthbertson, F.M. Ashcroft, T. Ezaki, and D.A. Doyle. 2003. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 300:1922–1926. [DOI] [PubMed] [Google Scholar]
- Kurata, H.T., L.R. Phillips, T. Rose, G. Loussouarn, S. Herlitze, H. Fritzenschaft, D. Enkvetchakul, C.G. Nichols, and T. Baukrowitz. 2004. Molecular basis of inward rectification: polyamine interaction sites located by combined channel and ligand mutagenesis. J. Gen. Physiol. 124:541–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaeus, M.J., M. Vamvouka, P.J. Focia, and A. Gross. 2005. Structural basis of TEA blockade in a model potassium channel. Nat. Struct. Mol. Biol. 12:454–459. [DOI] [PubMed] [Google Scholar]
- Lopatin, A.N., E.N. Makhina, and C.G. Nichols. 1994. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 372:366–369. [DOI] [PubMed] [Google Scholar]
- Lopatin, A.N., E.N. Makhina, and C.G. Nichols. 1995. The mechanism of inward rectification of potassium channels: “long-pore plugging” by cytoplasmic polyamines. J. Gen. Physiol. 106:923–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loussouarn, G., E.N. Makhina, T. Rose, and C.G. Nichols. 2000. Structure and dynamics of the pore of inwardly rectifying K(ATP) channels. J. Biol. Chem. 275:1137–1144. [DOI] [PubMed] [Google Scholar]
- Loussouarn, G., L.J. Marton, and C.G. Nichols. 2005. Molecular basis of inward rectification: structural features of the blocker defined by extended polyamine analogs. Mol. Pharmacol. 68:298–304. [DOI] [PubMed] [Google Scholar]
- Loussouarn, G., L.R. Phillips, R. Masia, T. Rose, and C.G. Nichols. 2001. Flexibility of the Kir6.2 inward rectifier K+ channel pore. Proc. Natl. Acad. Sci. USA. 98:4227–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Z. 2004. Mechanism of rectification in inward-rectifier K+ channels. Annu. Rev. Physiol. 66:103–129. [DOI] [PubMed] [Google Scholar]
- Lu, Z., and R. MacKinnon. 1994. Electrostatic tuning of Mg2+ affinity in an inward-rectifier K+ channel. Nature. 371:243–246. [DOI] [PubMed] [Google Scholar]
- Makary, S.M., T.W. Claydon, D. Enkvetchakul, C.G. Nichols, and M.R. Boyett. 2005. A difference in inward rectification and polyamine block and permeation between the Kir2.1 and Kir3.1/Kir3.4 K+ channels. J. Physiol. 568:749–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, C.G., and A.N. Lopatin. 1997. Inward rectifier potassium channels. Annu. Rev. Physiol. 59:171–191. [DOI] [PubMed] [Google Scholar]
- Nishida, M., and R. MacKinnon. 2002. Structural basis of inward rectification: cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 A resolution. Cell. 111:957–965. [DOI] [PubMed] [Google Scholar]
- Pearson, W.L., and C.G. Nichols. 1998. Block of the Kir2.1 channel pore by alkylamine analogues of endogenous polyamines. J. Gen. Physiol. 112:351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegan, S., C. Arrabit, W. Zhou, W. Kwiatkowski, A. Collins, P.A. Slesinger, and S. Choe. 2005. Cytoplasmic domain structures of Kir2.1 and Kir3.1 show sites for modulating gating and rectification. Nat. Neurosci. 8:279–287. [DOI] [PubMed] [Google Scholar]
- Phillips, L.R., D. Enkvetchakul, and C.G. Nichols. 2003. Gating dependence of inner pore access in inward rectifier K+ channels. Neuron. 37:953–962. [DOI] [PubMed] [Google Scholar]
- Phillips, L.R., and C.G. Nichols. 2003. Ligand-induced closure of inward rectifier Kir6.2 channels traps spermine in the pore. J. Gen. Physiol. 122:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, H.G., and Z. Lu. 2005. Mechanism of the voltage sensitivity of IRK1 inward-rectifier K+ channel block by the polyamine spermine. J. Gen. Physiol. 125:413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, H.G., Y. Xu, and Z. Lu. 2005. Evidence for sequential ion-binding loci along the inner pore of the IRK1 inward-rectifier K+ channel. J. Gen. Physiol. 126:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng, S., T. Ferrigni, and C.G. Nichols. 1997. Control of rectification and gating of cloned KATP channels by the Kir6.2 subunit. J. Gen. Physiol. 110:141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova, M., and Z. Lu. 1998. Coupled ion movement underlies rectification in an inward-rectifier K+ channel. J. Gen. Physiol. 112:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp, S., P. Proks, S.J. Tucker, and F.M. Ashcroft. 1998. Molecular analysis of ATP-sensitive K channel gating and implications for channel inhibition by ATP. J. Gen. Physiol. 112:333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible, B.A., M. Taglialatela, E. Ficker, and A.M. Brown. 1994. Gating of inwardly rectifying K+ channels localized to a single negatively charged residue. Nature. 371:246–249. [DOI] [PubMed] [Google Scholar]
- Yang, J., M. Yu, Y.N. Jan, and L.Y. Jan. 1997. Stabilization of ion selectivity filter by pore loop ion pairs in an inwardly rectifying potassium channel. Proc. Natl. Acad. Sci. USA. 94:1568–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, M., J.H. Morais-Cabral, S. Mann, and R. MacKinnon. 2001. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature. 411:657–661. [DOI] [PubMed] [Google Scholar]