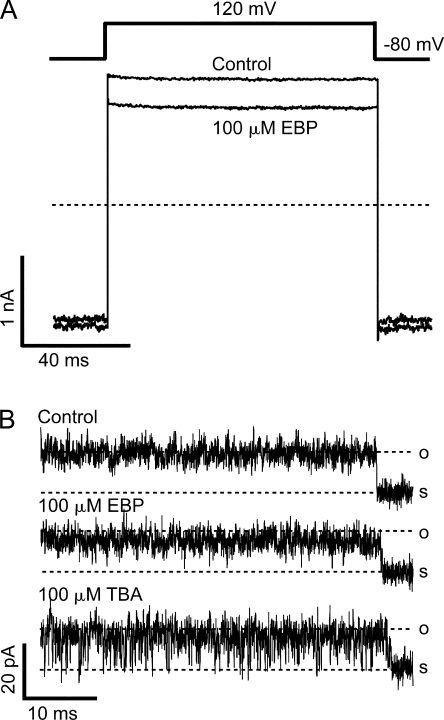
Figure 12.
EBP block of the S6/2-KcsA channels demonstrates a reduced apparent affinity and an enhanced dissociation rate. (A) Macroscopic currents were recorded from an inside-out patch expressing the S6/2-KcsA mutant channels in response to a depolarization of membrane potential to 120 mV before (control) and after the application of 100 μM EBP. Currents were not leak subtracted by P/4 protocol because channels don't close even at very negative potentials. Instead, current recorded in the presence of 20 mM TBA from the same patch was subtracted from the traces to remove most of the leak and capacitance transient. TBA at this concentration blocks nearly 100% of mutant currents, as suggested in Fig. 11 (A and D). (B) Single channel recording of an S6/2-KcsA channel in the absence (control) and presence of 100 μM EBP or 100 μM TBA.