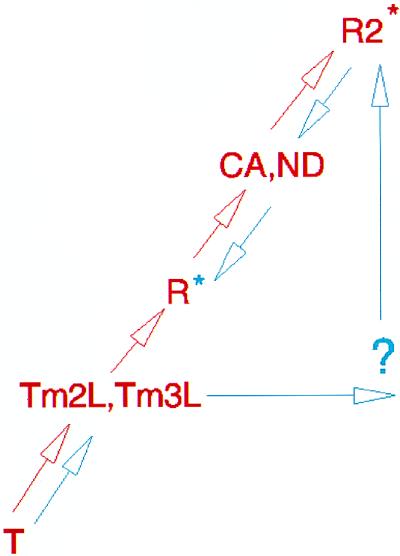
Figure 6.
Schematic representation of the proposed quaternary pathway of hemoglobin’s transition from the unliganded to liganded state. T, unliganded quaternary state; Tm2L, fully liganded, inter-β-subunit trimesoyl-crosslinked hemoglobin, α2β1Tm82β (22); Tm3L, fully liganded, intra- and inter-β-subunit trimesoyl-crosslinked hemoglobin, α2β1,82Tm82β (22); R, fully liganded, high-phosphate R state hemoglobin; CA, fully liganded, crosslinked hemoglobin, α2β82CA82β (described here); ND, fully liganded, crosslinked hemoglobin, α2β82ND82β (described here); R2, fully liganded R2 state. Two alternative paths are presented: one in which the R state is an intermediate and the R2 state is the final liganded state (red) and a second in which the R2 state is an intermediate and the R state is the final liganded state (blue). For each, an appropriate colored asterisk, blue or red, marks the final liganded state. The quaternary trajectory, as supported by the crystal structures of four intermediate quaternary structures (Tm2L, Tm3L, CA, and ND), is consistent only with the pathway depicted in red.