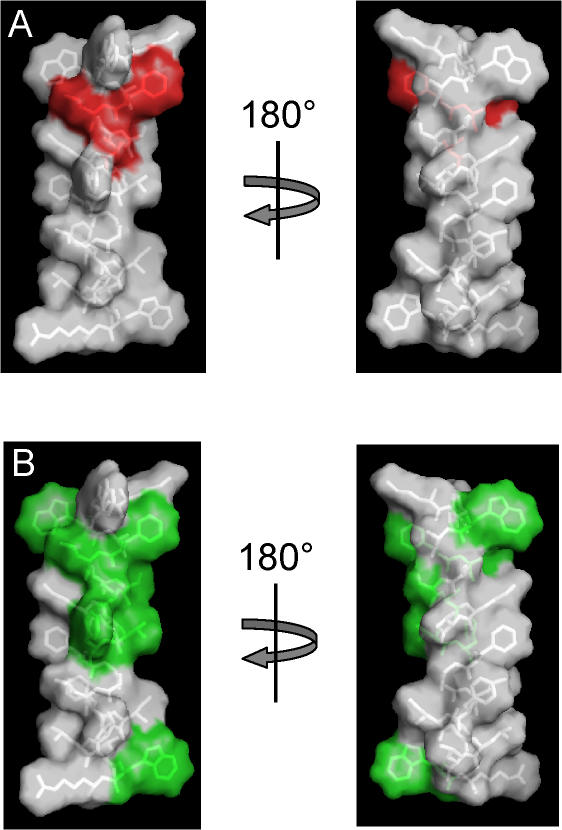
Figure 8.
Sequence that includes the S0 segment modeled as an α helix. Met-21 through Arg-44 were built as a helix using Pymol (Delano Scientific), with side chain rotamers selected to minimize steric clashes within the helix. (A) Side chains Phe-25, Leu-26, and Ser-29, corresponding to the positions of tryptophan mutants that give rise to large G-V shifts, are colored red. These are clustered on one face of the α helix. (B) Sidechains that are identical among slo1 orthologues (Figure 1), colored green. Except for Trp-23, which is one of the native tryptophans that flanks the putative transmembrane segment, all of the highly conserved side chains lie on one face of the helix.