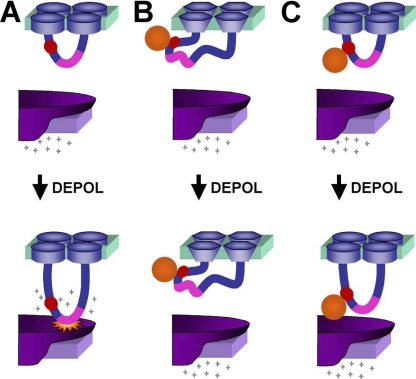
Figure 8.
Possible mechanisms for how binding of streptavidin to the α1S II-III loop could interfere with EC coupling. (A) During normal EC coupling, membrane depolarization induces a conformational change in the α1S II–III loop, which causes the activation of RyR1 (indicated by “starburst”) and release of calcium. The inserted BAD (red circle) is proximal to the “critical domain” (pink) and does not interfere with these events. (B) Binding of streptavidin could globally distort α1S such that the channel protein becomes nonfunctional. This possibility appears to be excluded since streptavidin binding did not affect the amplitude or gross voltage dependence of calcium currents. (C) Binding of streptavidin could interfere with the RyR1-activating conformational change of the II–III loop or another cytoplasmic portion of the DHPR.