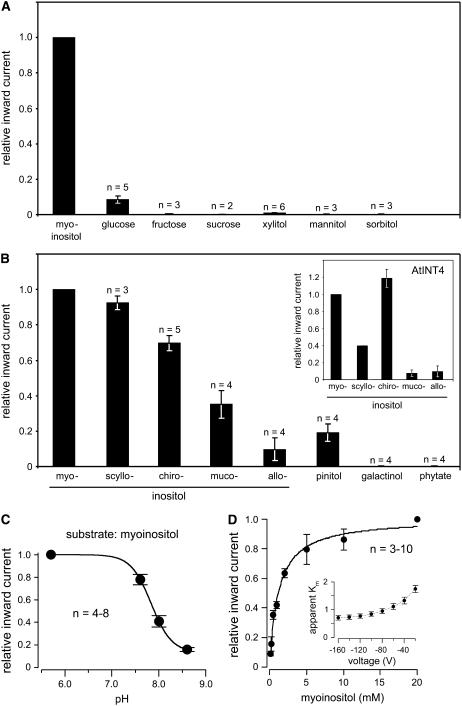
Figure 4.
Quantitative analyses of the substrate specificity, pH dependence, and Km myoinositol of recombinant AtINT2 in Xenopus oocytes. A, Quantitative analysis of inward currents elicited by different potential substrates of AtINT2 showing high specificity for inositol and a marginal capacity to transport Glc. B, Relative inward currents elicited by different inositol epimers and derivatives in AtINT2-expressing or AtINT4-expressing (inset) oocytes. C, pH dependence of myoinositol-elicited inward currents. D, The Km value of AtINT2 for myoinositol was determined by analyzing H+ currents in the presence of different myoinositol concentrations. Normalized currents were plotted against the substrate concentration. Data were fitted with Michaelis-Menten-type kinetics. The voltage dependence of the Km values is shown in the inset. Apparent Km values ranged from 0.7 ± 0.08 at −160 mV to 1.75 ± 0.13 at −20 mV (n = 5). Myoinositol-dependent H+ currents were determined at a membrane potential of −60 mV (A–D) or at voltages indicated (inset in D). Results ± se are presented.