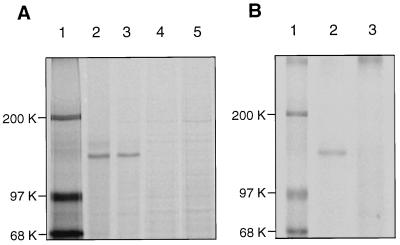
Figure 1.
Posttranslational modification of basonuclin in keratinocytes. (A) Reduction of electrophoretic mobility. Two half-confluent cultures were washed twice with 10 mM Hepes buffer, pH 7.5, containing 150 mM sodium chloride (HBS), starved for 30 min in the Met- and Cys-free medium, and labeled for 2 hr with [35S]methionine (1,000 Ci/mmol) at a concentration of 0.125 mCi/ml of culture medium. One of the labeled cultures was washed twice with Tris-buffered saline and immediately dissolved in Tris⋅SDS buffer. The other culture was washed with medium containing unlabeled methionine and cysteine to prevent further incorporation of [35S]methionine, incubated for 3 hr in that medium, washed, and dissolved in Tris/SDS buffer. Immunoprecipitation was carried out with antiserum. The mobility of the modified basonuclin after pulse–chase (lane 3) is slightly reduced in comparison with that of freshly synthesized basonuclin (lane 2). Neutralization of the antiserum with 0.3 mg/ml of the unlabeled recombinant basonuclin (HUB2) eliminated all basonuclin bands (lanes 4 and 5). Lane 1, molecular mass markers. (B) Phosphorylation of basonuclin. A quarter-confluent culture was washed twice with HBS and starved for phosphate for 18 hr. The culture, then about 50% confluent, was labeled for 5 hr with 32Pi (1 Ci/mmol) at a concentration of 0.125 mCi/ml of culture medium. The culture was washed twice with TBS, lysed immediately in Tris⋅SDS buffer, and the basonuclin was immuno-precipitated and resolved by electrophoresis. The basonuclin is clearly labeled with 32P (lane 2). Preliminary neutralization of the antiserum with unlabeled basonuclin eliminated the 32P-labeled basonuclin band (lane 3). Lane 1, molecular mass markers.