Abstract
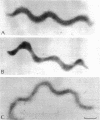
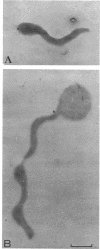
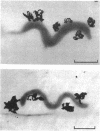
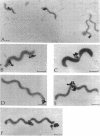
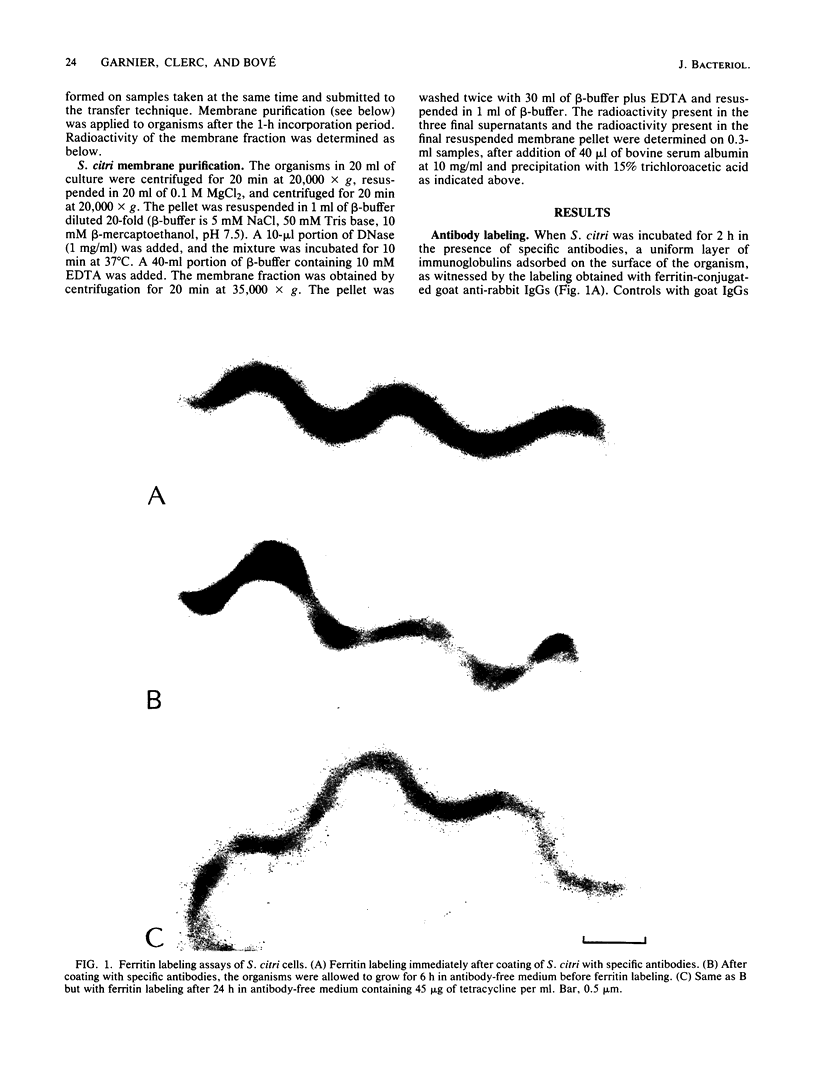
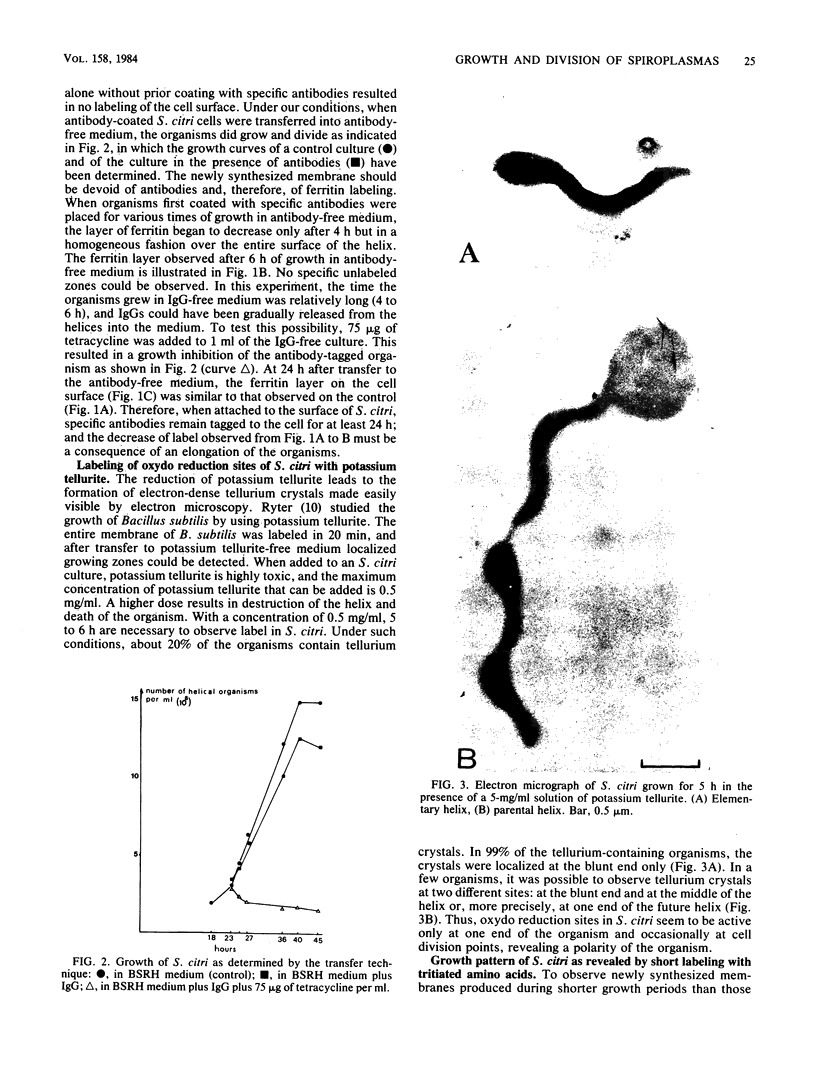
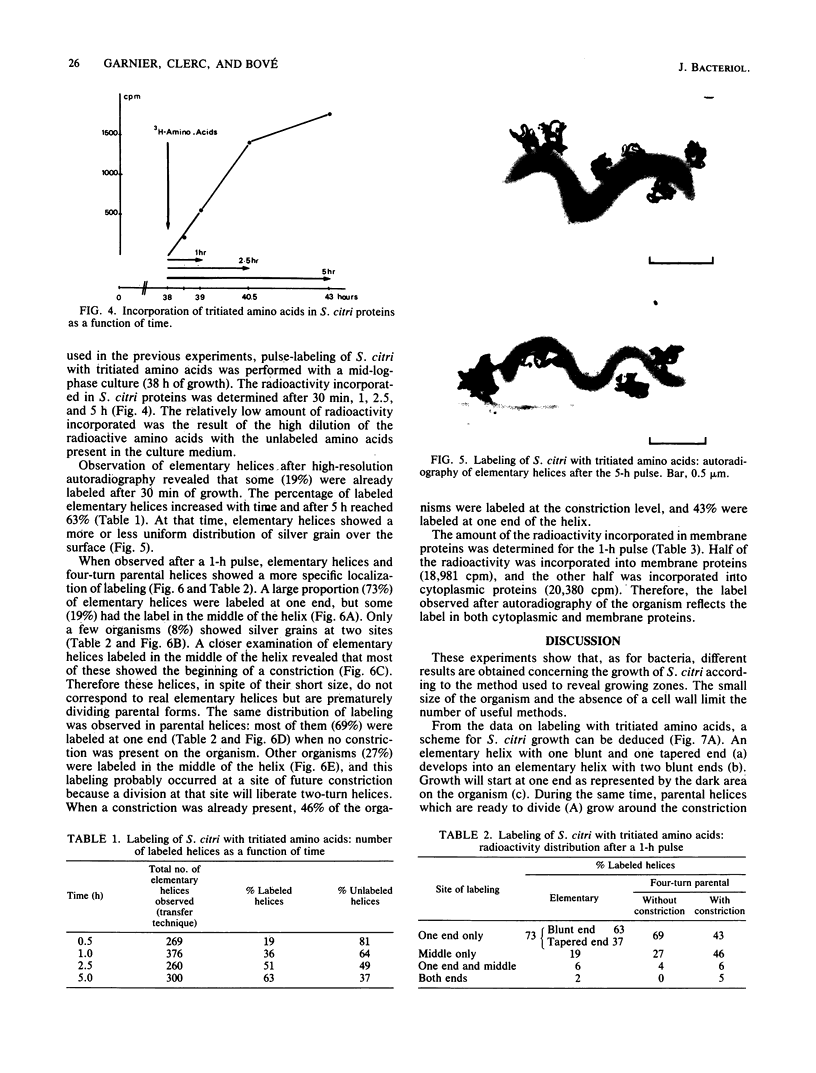
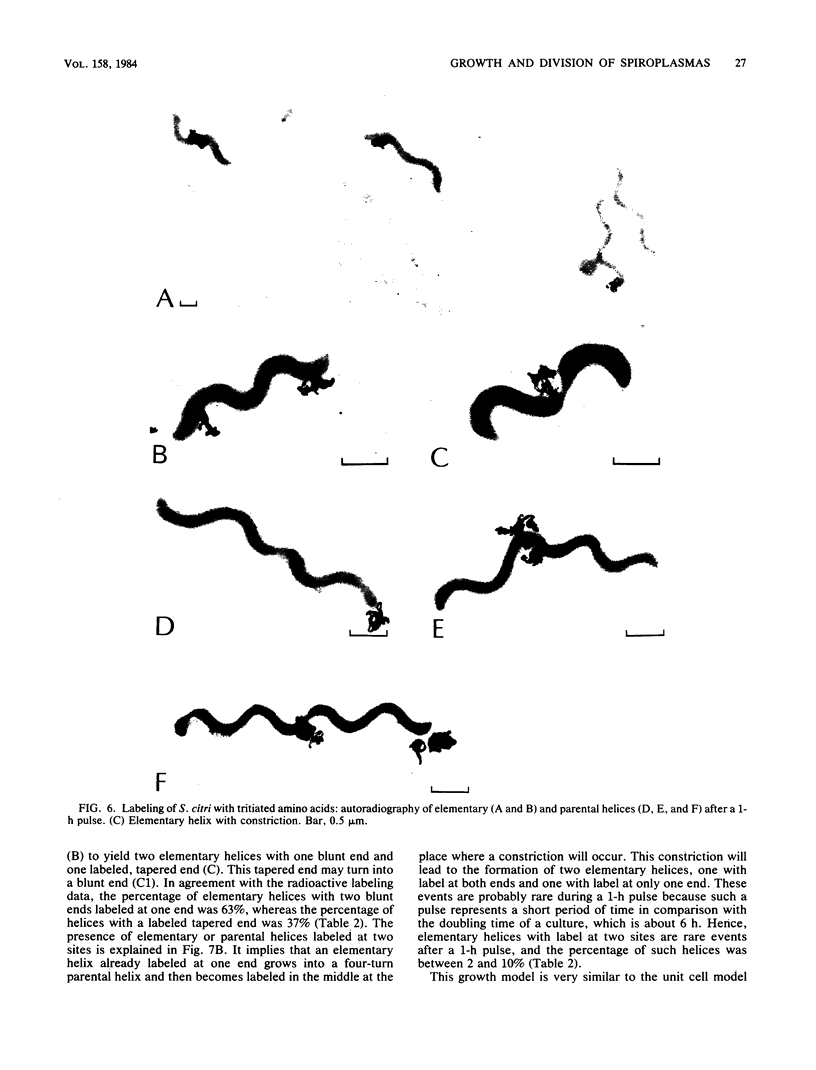
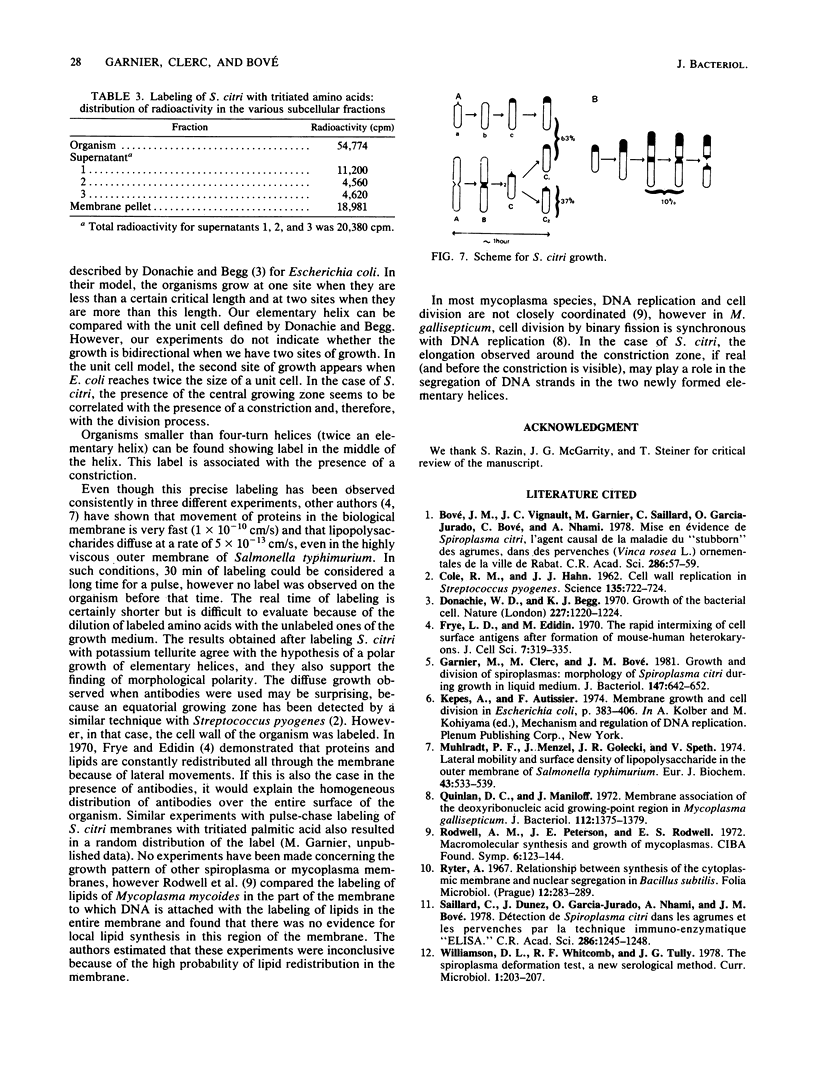
The smallest viable cell of Spiroplasma citri is a two-turn helix (elementary helix). This elementary helix grows into longer parental cells, which then divide by constriction. The helical morphology is conserved during this process. The growth pattern of S. citri membranes has been investigated by different methods of membrane labeling. When labeling is done with specific antibodies, a diffuse growth of the membrane is observed. On the contrary, pulse-labeling of the membrane with tritiated amino acids reveals a polar growth of the organism. Finally, labeling of oxydo reduction sites with potassium tellurite also indicates a polarity in the organism. These results are discussed, and a scheme for spiroplasma growth is proposed.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COLE R. M., HAHN J. J. Cell wall replication in Streptococcus pyogenes. Science. 1962 Mar 2;135(3505):722–724. doi: 10.1126/science.135.3505.722. [DOI] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J. Growth of the bacterial cell. Nature. 1970 Sep 19;227(5264):1220–1224. doi: 10.1038/2271220a0. [DOI] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Garnier M., Clerc M., Bove J. M. Growth and division of spiroplasmas: morphology of Spiroplasma citri during growth in liquid medium. J Bacteriol. 1981 Aug;147(2):642–652. doi: 10.1128/jb.147.2.642-652.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlradt P. F., Menzel J., Golecki J. R., Speth V. Lateral mobility and surface density of lipopolysaccharide in the outer membrane of Salmonella typhimurium. Eur J Biochem. 1974 Apr 16;43(3):533–539. doi: 10.1111/j.1432-1033.1974.tb03440.x. [DOI] [PubMed] [Google Scholar]
- Quinlan D. C., Maniloff J. Membrane association of the deoxyribonucleic acid growing-point region in Mycoplasma gallisepticum. J Bacteriol. 1972 Dec;112(3):1375–1379. doi: 10.1128/jb.112.3.1375-1379.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A. Relationship between synthesis of the cytoplasmic membrane and nuclear segregation in Bacillus subtilis. Folia Microbiol (Praha) 1967;12(3):283–290. doi: 10.1007/BF02868745. [DOI] [PubMed] [Google Scholar]