Abstract
The melanocortin-2 (MC2) receptor accessory protein (MRAP) is required for trafficking of the G protein-coupled MC2 receptor to the plasma membrane. The mechanism of action and structure of MRAP, which has a single transmembrane domain, are unknown. Here, we show that MRAP displays a previously uncharacterized topology. Epitopes on both the N- and C-terminal ends of MRAP were localized on the external face of CHO cells at comparable levels. Using antibodies raised against N- and C-terminal MRAP peptides, we demonstrated that both ends of endogenous MRAP face the outside in adrenal cells. Nearly half of MRAP was glycosylated at the single endogenous N-terminal glycosylation site, and over half was glycosylated when the natural glycosylation site was replaced by one in the C-terminal domain. A mutant MRAP with potential glycosylation sites on both sides of the membrane was singly but not doubly glycosylated, suggesting that MRAP is not monotopic. Coimmunoprecipitation of differentially tagged MRAPs established that MRAP is a dimer. By selectively immunoprecipitating cell surface MRAP in one or the other orientation, we showed that MRAP homodimers are antiparallel and form a stable complex with MC2 receptor. In the absence of MRAP, MC2 receptor was trapped in the endoplasmic reticulum, but with MRAP, the MC2 receptor was glycosylated and localized on the plasma membrane, where it signaled in response to ACTH. MRAP acted specifically, because it did not increase surface expression of other melanocortin, β2-adrenergic, or TSH-releasing hormone receptors. MRAP is the first eukaryotic membrane protein identified with an antiparallel homodimeric structure.
Keywords: ACTH, G protein-coupled receptor, membrane, orientation
The G protein-coupled receptor (GPCR) family is the largest group of membrane proteins in the human genome. GPCRs respond to a wide array of signals and regulate numerous intracellular signaling pathways. They are characterized by an extracellular amino terminus that is usually glycosylated, seven transmembrane domains, and a cytoplasmic carboxyl terminus. After synthesis in the endoplasmic reticulum, GPCRs must move to the plasma membrane before they can signal in response to extracellular ligands.
In the adrenal gland, the melanocortin-2 (MC2) receptor, also referred to as the corticotropin (ACTH) receptor, is activated by the pituitary hormone ACTH and promotes glucocorticoid biosynthesis. The MC2 receptor is expressed primarily in the adrenal cortex and is positively coupled to adenylyl cyclase. The MC2 receptor is a member of the class A rhodopsin-like family and the smallest known GPCR. Individuals harboring inactivating mutations in the MC2 receptor suffer from familial glucocorticoid deficiency, or hereditary unresponsiveness to ACTH (1). Recently, it was discovered that familial glucocorticoid deficiency can also arise from mutations in an accessory protein required for ACTH signaling, the MC2 receptor accessory protein (MRAP) (2). Lack of functional MRAP, like the lack of an MC2 receptor, causes ACTH resistance and severe glucocorticoid deficiency that can be fatal if the condition is not recognized and treated.
Accessory proteins are required for the expression of some GPCRs. For example, odorant receptors cannot be expressed in cells unless RTP1, RTP2, or REEP1 is coexpressed (3). The GABAB1 receptor contains an endoplasmic reticulum retention signal in its cytoplasmic tail and must form a heterodimer with a GABAB2 receptor before the GABAB receptor can move to the cell surface (4). Receptor activity modifying proteins (RAMPs) facilitate trafficking and alter the ligand specificity of several class B GPCRs (5). MRAP is expressed in the adrenal cortex but is not found in commonly used model cells such as HEK293, COS, and CHO, and the MC2 receptor is not active when it is expressed in cells lacking MRAP. MRAP is not structurally related to any of the previously identified GPCR accessory proteins.
Mouse MRAP has a single predicted transmembrane domain at amino acids 37–59. The N-terminal and transmembrane regions of MRAP are highly conserved, whereas the C-terminal regions are highly divergent. The orientation of membrane proteins is governed in part by charge. The “positive-inside rule,” originally developed for bacterial proteins, dictates that the side of the protein with higher positive charge near the transmembrane region is more likely to face the cytoplasm (6). The difference in net charge between N- and C-terminal sequences flanking the transmembrane domain is a better indicator for eukaryotic proteins (7). Topology prediction programs such as TMHMM (8) suggest that mouse MRAP is a Type II integral membrane protein, i.e., it is oriented with its C terminus outside the cell and N terminus inside, and the C-terminal ends of epitope-tagged MRAPs have been detected on the surface of nonpermeabilized transfected cells (2, 9). Prediction of the orientation of single transmembrane proteins is unreliable (10), however, and TMHMM assigns a low preference for the Nin-Cout orientation for MRAP. MRAP does not have recognizable protein folding domains that can influence orientation (11).
We have studied how MRAP facilitates MC2 receptor expression and characterized the topology of MRAP. We show that MRAP is inserted in the plasma membrane as an antiparallel homodimer and that MRAP is required for the MC2 receptor to move out of the endoplasmic reticulum and undergo posttranslational processing.
Results
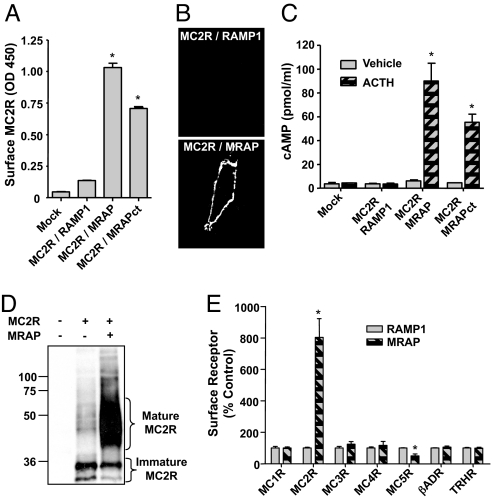
To explore how MRAP permits expression of functional MC2 receptor in heterologous cells, we expressed the MC2 receptor, tagged with HA-epitopes on its extracellular N terminus, with RAMP1 or MRAP. RAMP1 served as a negative control; it is a single transmembrane domain accessory protein for the calcitonin receptor-like receptor (5). When MC2 receptor was expressed with RAMP1 (Fig. 1 A and B) or alone (data not shown), the receptor was not detectable on the extracellular surface of nonpermeabilized cells in ELISA or immunofluorescence microscopy. In contrast, in the presence of MRAP, MC2 receptor could traffic to the plasma membrane. ACTH did not increase cAMP in cells expressing MC2 receptor and RAMP1, but increased cAMP dramatically in cells expressing MC2 receptor and MRAP (Fig. 1C), demonstrating that MRAP is required for MC2 receptor signaling.
Fig. 1.
MRAP promotes MC2 receptor maturation. (A–D) CHO cells were transfected with HA-MC2 receptor (MC2R), RAMP1, MRAP, or MRAPct. (A) Cell surface receptor detected by ELISA, using anti-HA antibody on fixed nonpermeabilized cells. (B) Surface staining of live cells with anti-HA antibody. (C) cAMP response to 100 nM ACTH. (D) Western blot of immunoprecipitated HA-MC2 receptor. (E) Cells were transfected with MRAP or RAMP1 and N-terminally HA-tagged melanocortin (MC) receptors 1–5, β2-adrenergic receptor, or TRH receptor. Surface receptors were quantified as in A, and values are normalized to the RAMP1 control for each receptor. Shown are the mean and range from two experiments, each containing two or three replicates. Control expression was significantly higher for MC5, β2-adrenergic, and TRH receptors than for MC1–4 receptors. Total receptor expression, measured by ELISA after addition of detergent, was within ±40% for all receptors except the MC4 receptor, which expressed at 20% the level of other receptors. *, P < 0.05 vs. mock-transfected.
MRAP remained functional when all but three residues of the C terminus were replaced with an epitope tag (MRAPct) (Fig. 1A and 2E), consistent with the lack of sequence conservation in this part of the protein. In fact, two splice variants of human MRAP differ completely in the C terminus, and both the 172-aa α and 102-aa β forms are functional (9).
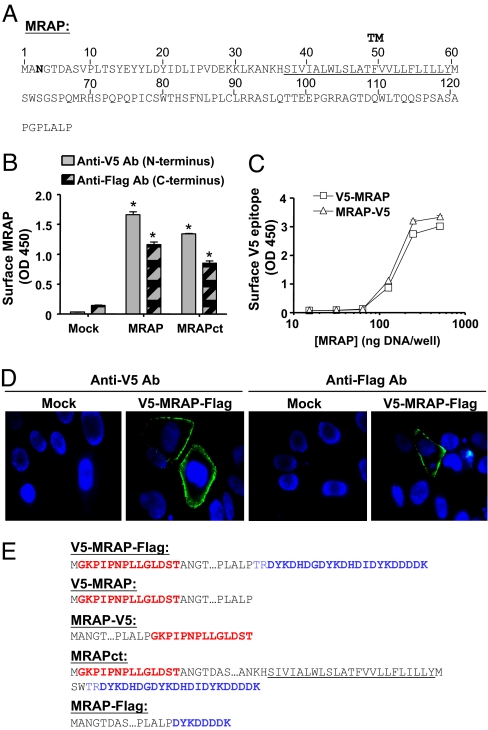
Fig. 2.
Transfected MRAP is inserted in the membrane in two opposite orientations. (A) Amino acid sequence of mouse MRAP. The underlined sequence is the predicted transmembrane domain, and the Asn shown in bold is the single natural potential N-linked glycosylation site. (B) Detection of both V5- and Flag-epitopes on the surface of nonpermeabilized CHO cells expressing V5-MRAP-Flag or MRAPct with the same tags measured by ELISA. (C) Detection of surface V5-epitope by ELISA in CHO cells expressing V5-MRAP or MRAP-V5. (D) Surface staining of live CHO cells expressing V5-MRAP-Flag with anti-V5 (Left) or anti-Flag (Right) antibodies (shown in green); nuclei are counterstained in blue. (E) Epitope-tagged MRAP constructs. V5 epitopes are shown in red, and Flag epitopes are shown in blue.
To determine whether MRAP altered posttranslational modification of the MC2 receptor, we expressed receptor with either RAMP1 or MRAP. Most receptor isolated from CHO cells lacking MRAP ran as two tight bands, presumably the nonglycosylated and core glycosylated forms expected in the endoplasmic reticulum. Receptor isolated from cells expressing MRAP was much more abundant and a great majority was in the mature glycosylated form (Fig. 1D). Deglycosylation collapsed all bands to the predicted size (data not shown). When MC2 receptor on the plasma membrane was isolated by adding anti-HA antibody to intact cells, nearly all receptor was heavily glycosylated (see Fig. 5C and below). The specificity of MRAP action was examined by coexpressing MRAP or RAMP1 with each of the five members of the melanocortin receptor family and the β2-adrenergic receptor, all coupled to Gs, and the TSH-releasing hormone (TRH) receptor, which is coupled to Gq. MRAP increased surface expression of only the MC2 receptor (Fig. 1E).
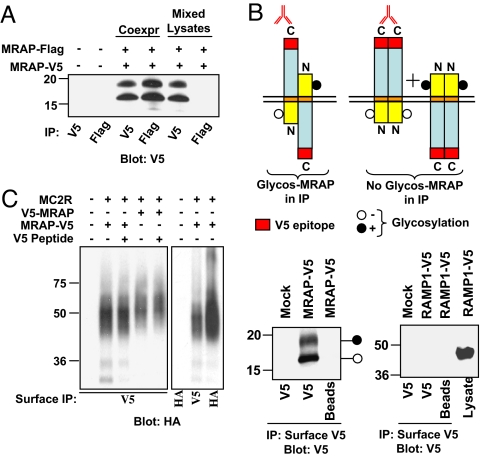
Fig. 5.
MRAP is an antiparallel homodimer interacting with MC2 receptor at the plasma membrane. (A) MRAP forms dimers. MRAP-V5 and MRAP-Flag were coexpressed in CHO cells. MRAP was immunoprecipitated with anti-V5 or anti-Flag antibody and immunoblotted with anti-V5 antibody. (B) MRAP dimers are antiparallel. Intact CHO cells expressing MRAP-V5 were incubated with anti-V5 antibody, washed, and lysed. Immunoprecipitates containing surface Nin-Cout MRAP were resolved on gels and blots probed with anti-V5 antibody. Control experiments confirmed that the upper band represents glycosylated MRAP (data not shown). Glycosylated MRAP must have been in Nout-Cin orientation. (C) MRAP forms a stable complex with MC2 receptor. Intact CHO cells expressing HA-MC2 receptor and either V5-MRAP or MRAP-V5 were incubated with anti-V5 antibody to isolate cell surface MRAP. Coprecipitated HA-MC2 receptor was detected by using anti-HA antibody. Adding 10 μg/ml V5-peptide to lysates caused little inhibition of immunoprecipitation, indicating that antibody did not dissociate. Coimmunoprecipitation of V5- and Flag-tagged MRAP and of HA-MC2 receptor and V5-tagged MRAP also worked in the reverse directions (data not shown).
To establish the topology of MRAP, we tagged MRAP with a V5-epitope on the N terminus and three Flag-epitopes on the C terminus (Fig. 2E) and expressed this V5-MRAP-Flag construct in CHO cells. Anti-V5 and anti-Flag antibodies both detected MRAP on the extracellular surface of nonpermeabilized cells (Fig. 2B), suggesting that either MRAP is inserted in the membrane in both Nout-Cin and Nin-Cout orientations or MRAP is a monotopic protein that partly traverses the membrane with both ends facing outward. Both N-terminal and C-terminal ends of MRAP were also detected on the plasma membrane by immunofluorescence microscopy in live cells (Fig. 2D). In both methods, signal was negligible in cells not expressing MRAP (Fig. 2 B and D). Intracellular epitopes were not detected in control studies, demonstrating that antibodies did not penetrate the cells in surface ELISA and immunofluorescence assays [supporting information (SI) Fig. 6]. Roy et al. (9) reported that both ends of human MRAP-β are visible on the surface of HEK293 cells after transient but not stable transfection, a result they attributed to overexpression.
To test whether dual orientation was influenced by overexpression or the use of different tags at the two ends of MRAP, we made additional constructs containing only a single V5 tag on one end or the other (Fig. 2E) and examined their orientations over a wide concentration range. Both the N-terminal and C-terminal V5-epitopes were localized on the extracellular surface of cells in nearly the same amounts, even at the lowest detectable level of MRAP expression (Fig. 2C). Dual topology of MRAP was unaffected by MC2 receptor expression (data not shown). MRAP retained its dual topology after deletion of most of the C terminus (Fig. 2B, MRAPct), establishing that the large carboxylterminal domain of MRAP does not specify topology. No MRAP was secreted into the culture medium, and no MRAP was extracted from membranes by 0.1 M sodium carbonate or 8 M urea (SI Fig. 7). Solubilization required detergent (1% Triton X-100 or 0.1% n-dodecyl-β-maltoside), confirming that MRAP is an intrinsic membrane protein.
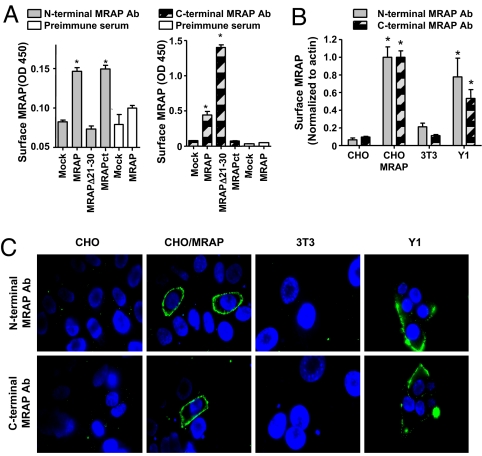
Because it was critical to determine whether endogenous MRAP displayed this unique dual topology, we raised rabbit antibodies against peptides representing residues 18–32 in the N terminus and residues 89–108 in the C terminus of MRAP. In CHO cells expressing RAMP1 or MRAP, reactivity with antibodies against native MRAP depended on the expression of MRAP in ELISA and immunofluorescence microscopy (Fig. 3 A–C). Antibody against the MRAP N terminus did not react with an MRAP mutant lacking residues 21–30, and antibody against the C terminus did not react with MRAPct (Fig. 3A). Preimmune sera were inactive in ELISAs and immunofluorescence microscopy (Fig. 3A). Mouse 3T3 cells that do not express MRAP (12) gave little signal, whereas adrenal Y1 cells that express endogenous MC2 receptor (13) gave strong signals in surface ELISAs (Fig. 3B) and immunofluorescence microscopy (Fig. 3C) with antibodies to both ends of MRAP. These results provide strong evidence that endogenous MRAP in adrenal cells has dual topology.
Fig. 3.
Endogenous MRAP is inserted in the membrane in two opposite orientations. (A) Specificity of rabbit antibodies to MRAP. Surface ELISA in CHO cells transfected with empty vector, MRAP, MRAPΔ21–30, or MRAPct, using affinity-purified rabbit N-terminal or C-terminal MRAP antibodies or preimmune sera. *, P < 0.05 versus mock-transfected. (B and C). Detection of N terminus and C terminus of MRAP on the plasma membrane. (B) Surface ELISA on CHO cells with or without transfected MRAP, mouse 3T3 fibroblasts, or mouse adrenal Y1 cells, using affinity-purified rabbit antisera to MRAP. (C) Live cell immunofluorescence microscopy of cells stained with affinity-purified rabbit anti-MRAP antibodies and monoclonal anti-actin. MRAP is shown in green, nuclei are counterstained in blue, and actin signal (shown in red) is undetectable (see also SI Fig. 6). *, P < 0.05 vs. mock-transfected.
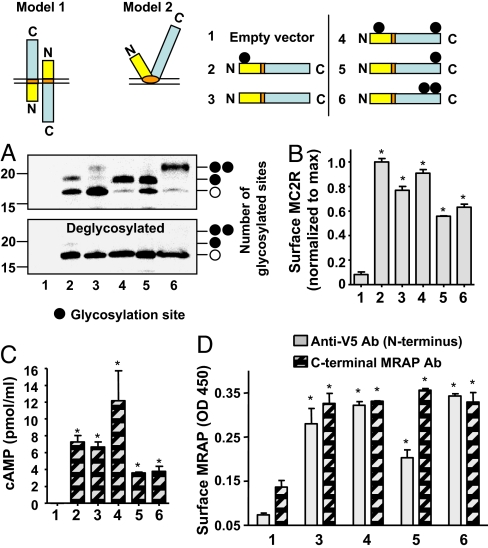
Evidence for dual orientation was also obtained from analysis of MRAP glycosylation patterns. Wild-type MRAP has a single potential N-linked glycosylation site on Asn-3 (Fig. 2A). To determine whether this site was used, we analyzed MRAP before and after deglycosylation. Close to half of MRAP ran at 19 kDa; this 19-kDa band collapsed to 17-kDa, the size of mouse MRAP with tags, after deglycosylation with PNGaseF (Fig. 4A, lane 2). When Asn-3 was mutated to Gln (MRAP-N3Q), only the 17-kDa nonglycosylated form was detected (Fig. 4, lane 3). Thus, a considerable fraction of wild type is normally glycosylated.
Fig. 4.
MRAP spans the membrane in two orientations. (A) CHO cells transfected with different glycosylation mutants of V5-tagged MRAP were lysed and MRAP immunoprecipitated with anti-V5 antibody and deglycosylated or not with PNGaseF. Blots were probed with anti-V5 antibody. When surface MRAP was selectively isolated (as described for Fig. 5B), approximately equal amounts of glycosylated and nonglycosylated MRAP were seen with MRAP-N3Q/Q96N/P88T. (B–D) Glycosylation mutants of MRAP are functional and display dual topology. CHO cells were transfected with empty vector, HA-MC2 receptor, and wild-type or V5-tagged mutant MRAP constructs. (B) Surface ELISA of HA-MC2 receptor (MC2R). (C) cAMP response to 100 nM ACTH. (D) Surface ELISA of N-terminal MRAP detected with anti-V5 antibody and C-terminal MRAP detected with affinity-purified rabbit anti-C-terminal MRAP antibody. *, P < 0.05 versus mock-transfected.
We designed a protein with a single potential glycosylation site on its C terminus by inserting a glycosylation site at position 96 of MRAP-N3Q (MRAP-N3Q/Q96N). More than half of the MRAP-N3Q/Q96N was glycosylated (Fig. 4A, lane 5). Because glycosylation takes place exclusively in the lumen of the endoplasmic reticulum, that both the wild-type MRAP (N-terminal glycosylation) and mutant MRAP-N3Q/Q96N (C-terminal glycosylation) are glycosylated provides further support for dual orientation.
We characterized an MRAP mutant with glycosylation sites in both the N-terminal and C-terminal sides of the transmembrane domain, MRAP-Q96N. We expected that if MRAP were a monotopic protein with both ends facing outside, some fraction of MRAP-Q96N would be glycosylated at both sites and run more slowly on gels. Although almost all MRAP-Q96N was glycosylated (Fig. 4A, lane 4), none ran behind singly glycosylated MRAP, consistent with the idea that only one site per molecule is used. To confirm that glycosylation at two sites would lead to a detectable up-shift, we showed that a mutant with two glycosylation sites, MRAP-N3Q/Q96N/P88T, was readily distinguished from singly glycosylated MRAP (Fig. 4A, lane 6). Deglycosylation collapsed all of the MRAP proteins to a single band. These findings support the dual topology model 1 depicted in Fig. 4. Interestingly, all of the glycosylation mutants displayed dual orientation (Fig. 4D) and promoted MC2 receptor expression and signaling (Fig. 4 B and C).
To determine whether MRAP forms homodimers, we coexpressed MRAP-V5 with MRAP-Flag, lysed cells and immunoprecipitated with either anti-V5 or anti-Flag antibody. A comparable amount of MRAP was detected after immunoprecipitation with either antibody, indicating that the vast majority of MRAP is in dimers or higher oligomers (Fig. 5A).
Two configurations are consistent with the finding that MRAP forms homodimers and displays dual orientation. MRAP could form antiparallel dimers, or MRAP could be present as a mixture of parallel homodimers with opposite orientation (Fig. 5B). To distinguish between these, we transfected CHO cells with MRAP-V5 and added anti-V5 antibody to intact cells, allowing only MRAP-V5 with the V5-epitope facing outward (Nin-Cout) to immunoprecipitate. When MRAP is in the Nin-Cout orientation, the unique glycosylation site at Asn-3 faces the cytoplasm and the molecule cannot be glycosylated. Immunoprecipitates of Nin-Cout MRAP-V5 contained both glycosylated and nonglycosylated MRAP, showing that nonglycosylated Nin-Cout MRAP was tightly bound to glycosylated Nout-Cin MRAP (Fig. 5B). In controls, cells were transfected with RAMP1-Venus-V5 (RAMP1-V5), which has an Nout-Cin orientation. RAMP1-Venus-V5 was not immunoprecipitated, showing that anti-V5 antibody did not react with intracellular V5-epitope. These results provide strong evidence that MRAP forms antiparallel homodimers.
To learn whether antiparallel MRAP dimers interact with MC2 receptors, we coexpressed HA-tagged receptors with either MRAP-V5 or V5-MRAP. Nin-Cout MRAP was selectively immunoprecipitated from cells expressing MRAP-V5, as described above, and Nout-Cin MRAP was selectively isolated from cells expressing V5-MRAP. Similar amounts of MC2 receptor coimmunoprecipitated with both Nout-Cin and Nin-Cout MRAPs (Fig. 5C), showing that receptor on the plasma membrane is in a tight complex with MRAP in both orientations. Because essentially all MRAP is in dimers, receptor interacts with antiparallel MRAP. ACTH did not alter MRAP-MC2 receptor interactions (data not shown).
Discussion
In this report, we have demonstrated that MRAP is required in the earliest stages of MC2 receptor processing and found that the MRAP protein has a previously uncharacterized structure. Without MRAP, MC2 receptor is either nonglycosylated or core glycosylated. Because the later steps of N-linked glycosylation take place in the Golgi apparatus, the lack of mature glycosylated receptor is consistent with the receptor's retention in the endoplasmic reticulum. GPCRs trapped in the endoplasmic reticulum are subject to degradation by retrotranslocation and proteasomal degradation (14). It seems likely that in the absence of its accessory protein, the MC2 receptor is extensively degraded by this mechanism in the endoplasmic reticulum, accounting for its low concentration in cells. MRAP effects were selective, because it did not promote cell surface expression of other GPCRs.
In our experiments with HEK293 cells (data not shown) and in our studies and those of Metherell et al. (2) with CHO cells, surface expression of MC2 receptor required MRAP. In contrast, Roy et al. (9) reported that MC2 receptor was detectable at the surface of HEK293 cells but incompetent to signal when it was expressed alone. Perhaps some lines of HEK293 cells express a low level of MRAP and the endogenous MRAP is sufficient to allow MC2 receptor to reach the plasma membrane but insufficient to support receptor signaling.
The dual orientation of MRAP was an unexpected finding that is supported by several different experimental approaches. Both ends of MRAP were detected on the exoplasmic face of cells expressing MRAP with different tags at the N- and C-termini, and both ends were found on the outer surface when MRAP was tagged with the same epitope at one end or the other. In this case, quantification was possible, and approximately equal amounts of surface MRAP were oriented with the amino and carboxyl ends facing out. Dual orientation of endogenous MRAP in adrenal cells was also demonstrated, eliminating the possibility that dual topology resulted from overexpression or epitope tagging. These conclusions all rely on the ability of antibodies to detect surface but not intracellular MRAP in nonpermeabilized cells. Glycosylation studies that do not depend on these methods provided further support for dual orientation. N-glycosylation can only occur when the Asn-X-Ser/Thr motif faces the inside of the endoplasmic reticulum, which is topologically equivalent to the external face of the plasma membrane. MRAP was partially glycosylated at its natural glycosylation site on the amino terminal side of the transmembrane domain and partially glycosylated when this site was removed and an artifical one introduced on the carboxyl side. Together, these results provide compelling evidence that both ends of MRAP face outward.
Two models could be invoked to explain the dual topology of MRAP. MRAP could be a transmembrane protein inserted in opposite orientations, or MRAP could be a monotopic protein anchored to the plasma membrane through its hydrophobic region. An MRAP mutant with glycosylation sites on both sides of the hydrophobic domain was singly glycosylated, but none was glycosylated at both sites; dual glycosylation would be expected for a monotopic protein facing the extracellular space. Furthermore, known monotopic proteins, such as prostaglandin H synthase, have an amphipathic membrane helix (15), and no amphipathic helix is predicted in MRAP. The most reasonable interpretation of these findings is that MRAP is a transmembrane protein in both Nout-Cin and Nin-Cout orientations.
MRAP is, to our knowledge, the first single transmembrane protein identified that adopts a dual topology with significant fractions in each orientation. It is also the first single transmembrane protein reported to form antiparallel homodimers. A number of dual topology proteins with multiple transmembrane domains have been described. EmrE is a bacterial small multidrug resistance transporter capable of forming antiparallel homodimers, but there is uncertainty about whether EmrE exists as a dual topology protein in cells and whether the active form has a single or dual orientation (16, 17). Some eukaryotic proteins, such as ductin, are also reported to have dual topology (18). Single transmembrane proteins with dual topology have been engineered by manipulating the charge near the membrane in proteins that normally have a single orientation (19). Cytochrome P450–2E1, with one transmembrane segment, has 2% inserted in the membrane in an inverted orientation (20), and a small fraction of epoxide hydrolase has been reported to have an inverted topology (21).
An intriguing question is how MRAP, which contains no signal sequence, is synthesized and inserted into the membrane in two orientations. Glycosylation does not control topology, because MRAP orientation and function were the same when the single natural glycosylation site was removed, and MC2 receptor expression did not affect topology. MRAP may be synthesized with similar amounts in each orientation. Alternatively, Nout-Cin and Nin-Cout MRAPs may be made in different amounts, but the Nout-Cin/Nin-Cout MRAP dimer may be the most stable form and preferentially trafficked to the Golgi apparatus and the plasma membrane. Available data suggest that the antiparallel MRAP dimer is the structure that interacts with the MC2 receptor. In addition, we characterized numerous MRAP mutants in an effort to force orientation to one direction or the other. Only those mutants that displayed dual topology permitted MC2 receptor function (unpublished data).
We conclude that the MRAP accessory protein displays a unique structure. This single transmembrane protein is inserted in the membrane in both Nout-Cin and Nin-Cout orientations. MRAP is isolated in dimers or higher aggregates, and the MRAP dimer is antiparallel. The MC2 receptor is in a complex containing both Nout-Cin and Nin-Cout MRAP. MRAP permits the receptor to move from the endoplasmic reticulum to the plasma membrane, where MRAP dimers and the receptor remain stably associated. MRAP is the first eukaryotic protein identified with an antiparallel dimeric structure. It seems likely that other accessory proteins for GPCRs remain to be discovered and it will be of interest to determine whether other accessory proteins have the same unusual structure as MRAP.
Materials and Methods
Materials.
All five hMC receptors and hβ2-adrenergic receptors with three N-terminal HA tags were obtained from the Missouri S&T cDNA Resource Center, MRAP-Flag was obtained from A. Clark (University of London, London, U.K.), RAMP1 constructs were obtained from I. Dickerson (University of Rochester, Rochester, NY), and Y1 cells were obtained from B. Schimmer (University of Toronto, Toronto, Canada). The HA-tagged TRH receptor is described in ref. 23. Peptides and rabbit antibodies to MRAP were prepared by New England Peptide. Peptides from mMRAP [Ac-LDYIDLIPVDEKKLKC-amide (Cys added) and Ac-CLRRASLQTTEEPGRRAGTD-amide] were coupled via Cys to keyhole limpet hemocyanin. Antibodies were affinity-purified on Pierce Sulfolink columns. Antibodies were obtained from Invitrogen (monoclonal anti-V5), Sigma (monoclonal M2 anti-Flag), Covance (monoclonal HA11 anti-HA), Bio-Rad (HRP-coupled anti-mouse or anti-rabbit), Abcam (monoclonal anti-actin), or Molecular Probes (Alexa Fluor 488-, 546-, and 633-coupled anti-mouse or anti-rabbit). MRAP mutants were prepared by using Stratagene Quikchange and verified by sequencing.
Cell Culture and Transfection.
Cells were grown in DMEM/F-12 supplemented with 5% FBS for CHO cells; 10% calf serum for 3T3 cells; or 15% horse serum, 2.5% FBS, and antibiotics for Y1 cells. Plasmids were transiently transfected 24–48 h before experiments, using Fugene 6 (Roche).
Surface Epitope Detection by Fixed-Cell ELISA.
To measure epitopes on the extracellular side of the plasma membrane, cells in 12-well plates were washed with PBS, fixed for 10 min with 4% paraformaldehyde, washed, blocked in 5% milk in PBS, and processed for ELISA as described in ref. 22, using 1:5,000 monoclonal anti-V5, anti-Flag, and anti-HA antibodies, 1:250 rabbit N-terminal MRAP antibody, or 1:500 rabbit C-terminal MRAP antibody.
Live Cell Imaging.
Cells on glass coverslips were rinsed and incubated with primary antibodies (1:100 except for anti-actin antibody at 1:5,000) in F12 media with 20 mM Hepes and 5% goat serum for 1 h at 37°C, washed, and incubated with 1:100 secondary antibody and 3 μg/ml Hoechst 33342 for 5 min at room temperature. Secondary antibodies were Alexa Fluor 546 anti-mouse with anti-HA antibody, Alexa Fluor 488 anti-mouse with anti-V5 and anti-Flag antibodies, Alexa Fluor 488 anti-rabbit with rabbit anti-MRAP, and Alexa Fluor 633 anti-mouse with anti-actin antibody. A Nikon Diaphot inverted microscope with a 100×/1.3 N.A. oil objective, a Photometrics CoolSNAP ES camera, and appropriate filter sets from Chroma were used. Images were captured with Metamorph software from Universal Imaging and transferred to Powerpoint for labeling. Micrographs displayed in a group were exposed and processed identically.
Immunoprecipitation and Western Blotting.
For surface epitope immunoprecipitation, cells were washed, incubated with 1:2,000 immunoprecipitating antibody (anti-HA, anti-V5, or anti-Flag) in F12 media with 20 mM Hepes and 5% goat serum for 2 h at 4°C, washed, and lysed for 20 min at 4°C with 0.1% n-dodecyl-β-maltoside in PBS with protease inhibitors. Lysates were centrifuged, and immune complexes were collected with protein A/G beads at 4°C. To immunoprecipitate total cell MRAP or MC2 receptor, cells were lysed with 0.1% n-dodecyl-β-maltoside and centrifuged, and supernatants were incubated overnight at 4°C with 1:5,000 primary antibody and collected on protein A/G beads. Beads were washed three times, suspended in loading buffer with 100 mM DTT, boiled 5 min, and centrifuged. Proteins were resolved by SDS/PAGE on 10% (MC2 receptor) or 15% (MRAP and RAMP1) gels from Lonza. Western blotting and deglycosylation were performed as described in refs. 23 and 24, respectively.
cAMP Assay.
CHO cells in 12-well plates were incubated with 0.1 mM 3-isobutyl-1-methylxanthine and vehicle or 100 nM ACTH in Opti-MEM I for 20 min at 37°C. cAMP was assayed by using Assay Design's cAMP EIA Direct kit.
Statistics.
The significance of differences among groups was analyzed by ANOVA with Tukey's post hoc analysis. Experiments were performed at least twice, and points show the mean and SE of triplicates. Where not visible, error bars fell within symbol size.
Supplementary Material
ACKNOWLEDGMENTS.
This work was supported by National Institutes of Health Grant DK19974.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.v.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708916105/DC1.
References
- 1.Metherell LA, Chan LF, Clark AJ. The genetics of ACTH resistance syndromes. Best Pract Res Clin Endocrinol Metab. 2006;20:547–560. doi: 10.1016/j.beem.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Metherell LA, Chapple JP, Cooray S, David A, Becker C, Ruschendorf F, Naville D, Begeot M, Khoo B, Nurnberg P, et al. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet. 2005;37:166–170. doi: 10.1038/ng1501. [DOI] [PubMed] [Google Scholar]
- 3.Saito H, Kubota M, Roberts RW, Chi Q, Matsunami H. RTP family members induce functional expression of mammalian odorant receptors. Cell. 2004;119:679–691. doi: 10.1016/j.cell.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- 5.Sexton PM, Morfis M, Tilakaratne N, Hay DL, Udawela M, Christopoulos G, Christopoulos A. Complexing receptor pharmacology: modulation of family B G protein-coupled receptor function by RAMPs. Ann N Y Acad Sci. 2006;1070:90–104. doi: 10.1196/annals.1317.076. [DOI] [PubMed] [Google Scholar]
- 6.von Heijne G. The distribution of positively charged residues in bacterial inner membrane proteins correlates with the transmembrane topology. EMBO J. 1986;5:3021–3027. doi: 10.1002/j.1460-2075.1986.tb04601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartmann E, Rapoport TA, Lodish HF. Predicting the orientation of eukaryotic membrane-spanning proteins. Proc Natl Acad Sci USA. 1989;86:5786–5790. doi: 10.1073/pnas.86.15.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viklund H, Elofsson A. Best alpha-helical transmembrane protein topology predictions are achieved by using hidden Markov models and evolutionary information. Protein Sci. 2004;13:1908–1917. doi: 10.1110/ps.04625404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy S, Rached M, Gallo-Payet N. Differential regulation of the human adrenocorticotropin receptor [melanocortin-2 receptor (MC2R)] by human MC2R accessory protein isoforms alpha and beta in isogenic human embryonic kidney 293 cells. Mol Endocrinol. 2007;21:1656–1669. doi: 10.1210/me.2007-0041. [DOI] [PubMed] [Google Scholar]
- 10.Bernsel A, Von Heijne G. Improved membrane protein topology prediction by domain assignments. Protein Sci. 2005;14:1723–1728. doi: 10.1110/ps.051395305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denzer AJ, Nabholz CE, Spiess M. Transmembrane orientation of signal-anchor proteins is affected by the folding state but not the size of the N-terminal domain. EMBO J. 1995;14:6311–6317. doi: 10.1002/j.1460-2075.1995.tb00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu A, Choi KL, Wang Y, Permana PA, Xu LY, Bogardus C, Cooper GJ. Identification of novel putative membrane proteins selectively expressed during adipose conversion of 3T3–L1 cells. Biochem Biophys Res Commun. 2002;293:1161–1167. doi: 10.1016/S0006-291X(02)00354-6. [DOI] [PubMed] [Google Scholar]
- 13.Rainey WE, Saner K, Schimmer BP. Adrenocortical cell lines. Mol Cell Endocrinol. 2004;228:23–38. doi: 10.1016/j.mce.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 14.Petaja-Repo UE, Hogue M, Laperriere A, Bhalla S, Walker P, Bouvier M. Newly synthesized human delta opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J Biol Chem. 2001;276:4416–4423. doi: 10.1074/jbc.M007151200. [DOI] [PubMed] [Google Scholar]
- 15.Garavito RM. Membrane protein structures: the known world expands. Curr Opin Biotechnol. 1998;9:344–349. doi: 10.1016/s0958-1669(98)80005-0. [DOI] [PubMed] [Google Scholar]
- 16.Schuldiner S. When biochemistry meets structural biology: the cautionary tale of EmrE. Trends Biochem Sci. 2007;32:252–258. doi: 10.1016/j.tibs.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Rapp M, Seppala S, Granseth E, von Heijne G. Emulating membrane protein evolution by rational design. Science. 2007;315:1282–1284. doi: 10.1126/science.1135406. [DOI] [PubMed] [Google Scholar]
- 18.Dunlop J, Jones PC, Finbow ME. Membrane insertion and assembly of ductin: a polytopic channel with dual orientations. EMBO J. 1995;14:3609–3616. doi: 10.1002/j.1460-2075.1995.tb00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beltzer JP, Fiedler K, Fuhrer C, Geffen I, Handschin C, Wessels HP, Spiess M. Charged residues are major determinants of the transmembrane orientation of a signal-anchor sequence. J Biol Chem. 1991;266:973–978. [PubMed] [Google Scholar]
- 20.Neve EP, Ingelman-Sundberg M. Molecular basis for the transport of cytochrome P450 2E1 to the plasma membrane. J Biol Chem. 2000;275:17130–17135. doi: 10.1074/jbc.M000957200. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Q, von Dippe P, Xing W, Levy D. Membrane topology and cell surface targeting of microsomal epoxide hydrolase. Evidence for multiple topological orientations. J Biol Chem. 1999;274:27898–27904. doi: 10.1074/jbc.274.39.27898. [DOI] [PubMed] [Google Scholar]
- 22.Jones BW, Song GJ, Greuber EK, Hinkle PM. Phosphorylation of the endogenous thyrotropin-releasing hormone receptor in pituitary GH3 cells and pituitary tissue revealed by phosphosite-specific antibodies. J Biol Chem. 2007;282:12893–12906. doi: 10.1074/jbc.M610854200. [DOI] [PubMed] [Google Scholar]
- 23.Zhu CC, Cook LB, Hinkle PM. Dimerization and phosphorylation of thyrotropin-releasing hormone receptors are modulated by agonist stimulation. J Biol Chem. 2002;277:28228–28237. doi: 10.1074/jbc.M204221200. [DOI] [PubMed] [Google Scholar]
- 24.Jones BW, Hinkle PM. Beta-arrestin mediates desensitization and internalization but does not affect dephosphorylation of the thyrotropin-releasing hormone receptor. J Biol Chem. 2005;280:38346–38354. doi: 10.1074/jbc.M502918200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.