Fig. 5.
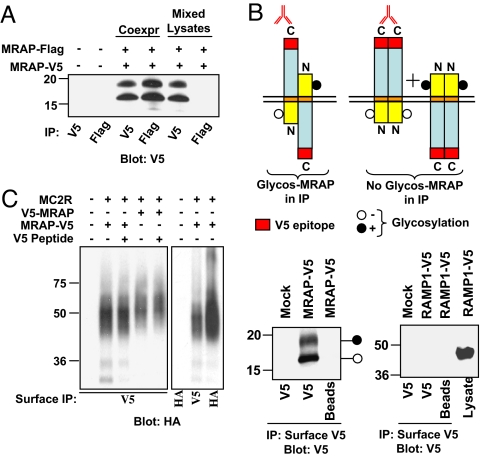
MRAP is an antiparallel homodimer interacting with MC2 receptor at the plasma membrane. (A) MRAP forms dimers. MRAP-V5 and MRAP-Flag were coexpressed in CHO cells. MRAP was immunoprecipitated with anti-V5 or anti-Flag antibody and immunoblotted with anti-V5 antibody. (B) MRAP dimers are antiparallel. Intact CHO cells expressing MRAP-V5 were incubated with anti-V5 antibody, washed, and lysed. Immunoprecipitates containing surface Nin-Cout MRAP were resolved on gels and blots probed with anti-V5 antibody. Control experiments confirmed that the upper band represents glycosylated MRAP (data not shown). Glycosylated MRAP must have been in Nout-Cin orientation. (C) MRAP forms a stable complex with MC2 receptor. Intact CHO cells expressing HA-MC2 receptor and either V5-MRAP or MRAP-V5 were incubated with anti-V5 antibody to isolate cell surface MRAP. Coprecipitated HA-MC2 receptor was detected by using anti-HA antibody. Adding 10 μg/ml V5-peptide to lysates caused little inhibition of immunoprecipitation, indicating that antibody did not dissociate. Coimmunoprecipitation of V5- and Flag-tagged MRAP and of HA-MC2 receptor and V5-tagged MRAP also worked in the reverse directions (data not shown).