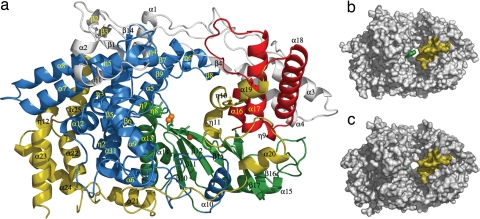
Fig. 1.
Structure of VP1 polymerase of IBDV. (a) View of a ribbon diagram showing the secondary structural elements explicitly labeled as follows: the N-terminal domain (gray) is folded in five helices (α1–α4 and the 310 helix η1), four β-strands (β1–β4), and three long loops. The VP1 fingers (blue) are organized in two regions. The first region is the inner fingers, consisting primarily of helices α5, α6, α9, α10, α11, and α12 surrounding and packed against the palm subdomain. The second region is the outer fingers, which include the following: (i) a five-stranded β-sheet (β1, β7, β9, β13, β14), with β1 contributed by the N-terminal domain, surrounded by a surface of exposed helices (η4, η5, α7, and α8); (ii) an adjacent region formed by helix η3 and the β-hairpin β5–β6 pointing out of the central cavity of the polymerase; and (iii) a loop formed by β8 and η6 (residues 321–335), which effectively extends toward the thumb domain. The palm subdomain (green) consists of a four-stranded β-sheet (β10, β11, β12, and β15) flanked by two α-helices (α13 and α14) and followed by an additional helix α15 and a long loop connecting the β16–β17 hairpin. The thumb subdomain (red) includes three α-helices (α16, α17, and α18) connected by long loops. Finally, the C-terminal domain (yellow; residues 671–804) includes four helices (α19, η10, η11, and α20), next to the thumb, followed by six α-helices (α21–α25 and η12), close to fingers that are connected by a long and flexible loop. (b) Surface representation of the IBDV VP1 in its apo form, showing the central cleft, which is partially covered by two protrusions that project from the B loop (green) and the α19-η10-η11 region (yellow) at the C-terminal domain. (c) Surface representation of the IBDV VP1 when complexed with the VP3 derived peptide.