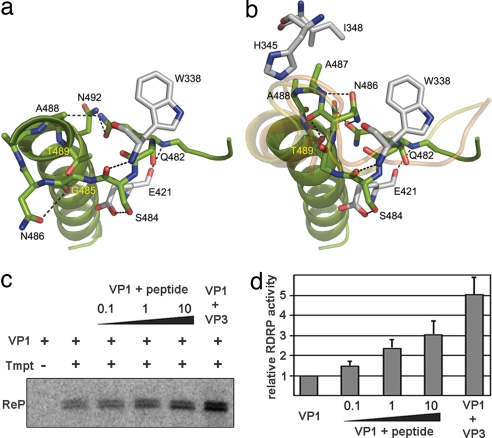
Fig. 3.
Structural rearrangements of the B loop and effects of the VP3 C-terminal peptide and the VP3 polypeptide on VP1 RDRP activity. (a and b) The closed conformation found in the structure of the isolated VP1 (a) and the open conformation found in the VP1–VP3 complex (b). The amino acids involved in intramolecular contacts that stabilize the two alternative conformations of the B loop are shown in sticks and explicitly labeled. The main chain tracing of the equivalent loop in FMDV and φ6 RDRPs correlates well with the open conformation found in the structure of the VP1–VP3 complex (yellow and orange ribbons in b, respectively). (c) Purified hVP1 was preincubated with either the VP3 C-terminal peptide (peptide) or purified hVP3 (VP3). Peptide/hVP1 molar ratios were 0.1:1 (0.1), 1:1 (1), and 10:1 (10), respectively. hVP3 was used at a 2:1 hVP3/hVP1 molar ratio. Samples were supplemented (+) or not (−) with a 544-nt ssRNA+ template (Tmpt). Labeling was performed by using [32P]UTP, and reaction products (ReP) were separated on 5% acrylamide TBE gels. (d) RDRP activity after preincubation with the VP3 C-terminal peptide and the VP3 polypeptide relative to VP1 alone, which was defined as 1. The mean value for each condition was obtained from three independent experiments.