Abstract
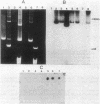
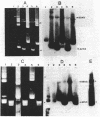
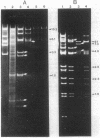
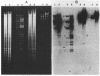
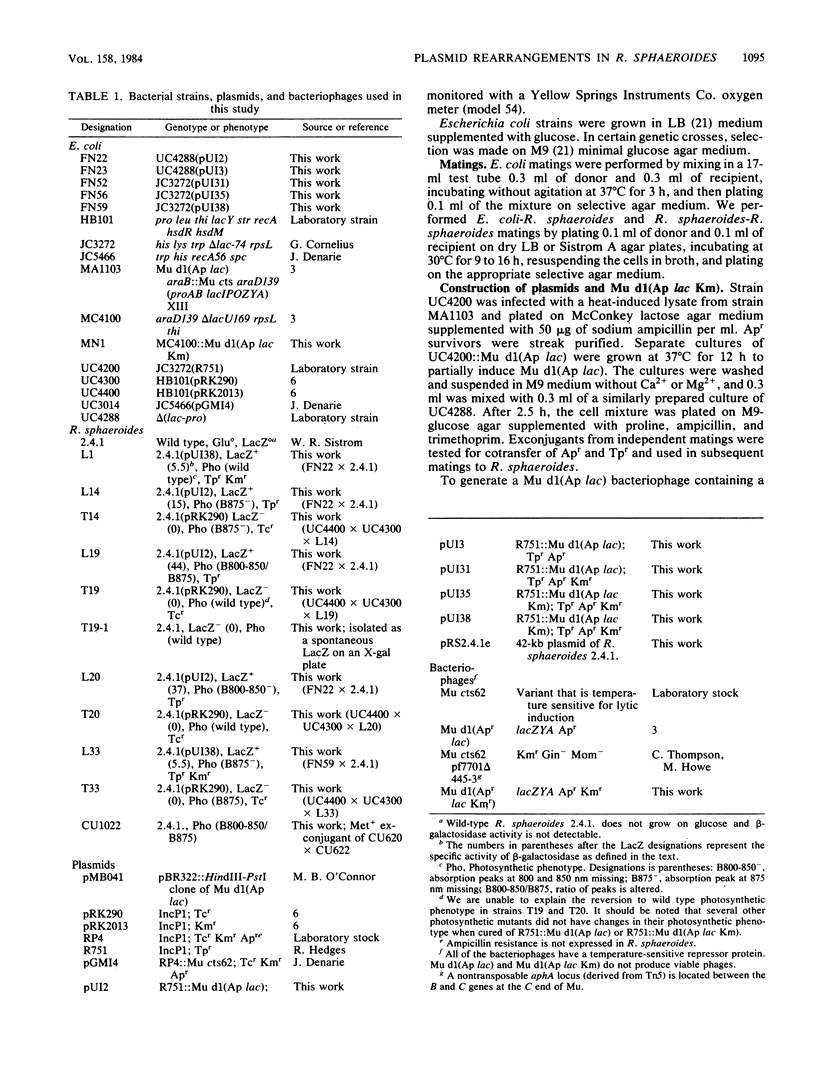
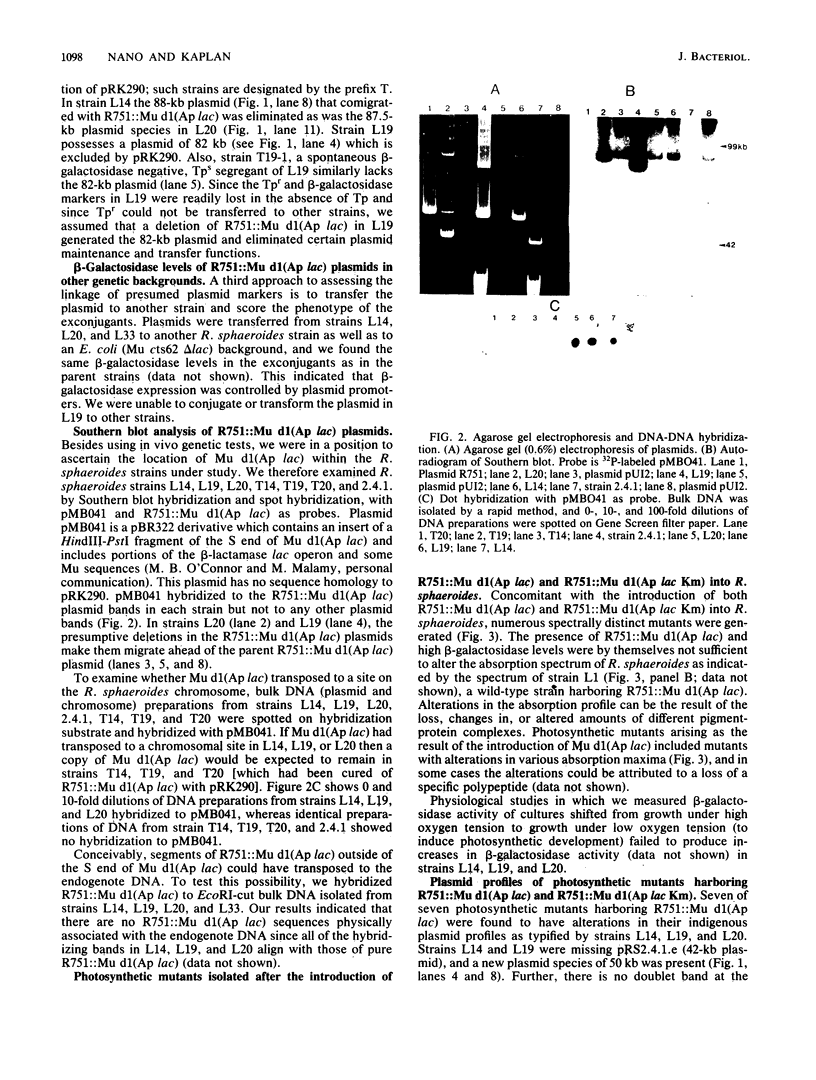
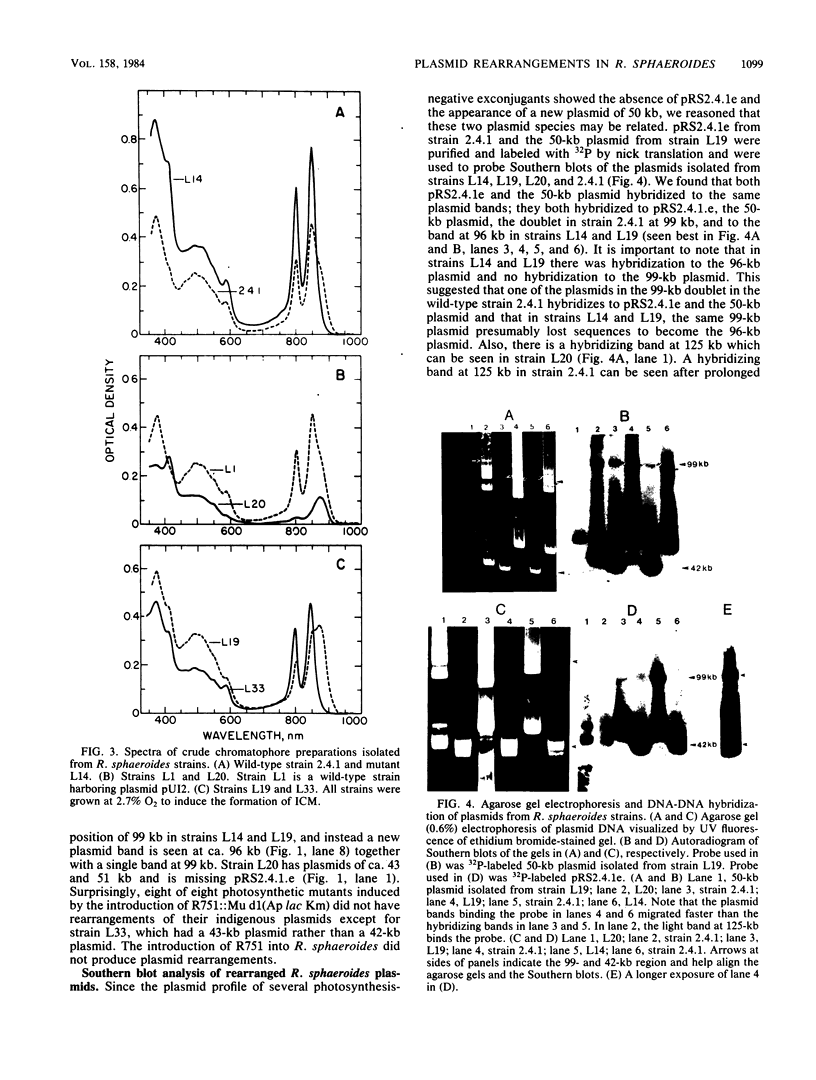
Mu d1(Ap lac) was introduced into the photosynthetic bacterium Rhodopseudomonas sphaeroides 2.4.1. via the R-plasmid R751 in an attempt to isolate fusion derivatives involving photosynthetic operons. The selection system is potentially very powerful since R. sphaeroides is normally Lac negative. Among the exconjugants, photosynthesis-deficient mutants were recovered, some of which had elevated beta-galactosidase levels. Among the mutants examined, beta-galactosidase expression was linked exclusively to R751 . Many of the photosynthesis-deficient mutants were found to have alterations in their indigenous plasmids which apparently involved the exchange of DNA from one plasmid to another. Southern blot analysis revealed that there are extensive DNA sequences which are shared by the two plasmids that are involved in the rearrangements and that no exogenous DNA sequences appear to be involved. It was further discovered that plasmid rearrangement is a general phenomenon which can occur spontaneously in R. sphaeroides 2.4.1 and shows a high correlation with a photosynthesis minus phenotype.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aagaard J., Sistrom W. R. Control of synthesis of reaction center bacteriochlorophyll in photosynthetic bacteria. Photochem Photobiol. 1972 Feb;15(2):209–225. doi: 10.1111/j.1751-1097.1972.tb06240.x. [DOI] [PubMed] [Google Scholar]
- Bukhari A. I., Ambrosio L. The invertible segment of bacteriophage Mu DNA determines the adsorption properties of Mu particles. Nature. 1978 Feb 9;271(5645):575–577. doi: 10.1038/271575a0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Bukhari A. I. The invertible DNA segments of coliphages Mu and P1 are identical. Virology. 1976 Oct 1;74(1):242–248. doi: 10.1016/0042-6822(76)90148-3. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic M. A., Zurkowski W., Rolfe B. G. Plasmids and stability of symbiotic properties of Rhizobium trifolii. J Bacteriol. 1982 Aug;151(2):560–568. doi: 10.1128/jb.151.2.560-568.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faelen M., Huisman O., Toussaint A. Involvement of phage Mu-1 early functions in Mu-mediated chromosomal rearrangements. Nature. 1978 Feb 9;271(5645):580–582. doi: 10.1038/271580a0. [DOI] [PubMed] [Google Scholar]
- Fornari C. S., Kaplan S. Genetic transformation of Rhodopseudomonas sphaeroides by plasmid DNA. J Bacteriol. 1982 Oct;152(1):89–97. doi: 10.1128/jb.152.1.89-97.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornari C. S., Watkins M., Kaplan S. Plasmid distribution and analyses in Rhodopseudomonas sphaeroides. Plasmid. 1984 Jan;11(1):39–47. doi: 10.1016/0147-619x(84)90005-2. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp D., Kahmann R., Zipser D., Broker T. R., Chow L. T. Inversion of the G DNA segment of phage Mu controls phage infectivity. Nature. 1978 Feb 9;271(5645):577–580. doi: 10.1038/271577a0. [DOI] [PubMed] [Google Scholar]
- Kutsukake K., Iino T. Inversions of specific DNA segments in flagellar phase variation of Salmonella and inversion systems of bacteriophages P1 and Mu. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7338–7341. doi: 10.1073/pnas.77.12.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNKRES K. D., RICHARDS F. M. THE PURIFICATION AND PROPERTIES OF NEUROSPORA MALATE DEHYDROGENASE. Arch Biochem Biophys. 1965 Mar;109:466–479. doi: 10.1016/0003-9861(65)90391-7. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L., Kaplan S. Plasmid transfer and expression in Rhodopseudomonas sphaeroides. Arch Biochem Biophys. 1978 Apr 15;187(1):229–234. doi: 10.1016/0003-9861(78)90028-0. [DOI] [PubMed] [Google Scholar]
- Nano F. E., Kaplan S. Expression of the transposable lac operon Tn951 in Rhodopseudomonas sphaeroides. J Bacteriol. 1982 Nov;152(2):924–927. doi: 10.1128/jb.152.2.924-927.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B. C., Hashimoto H., Rownd R. H. Cointegrate formation between homologous plasmids in Escherichia coli. J Bacteriol. 1982 Sep;151(3):1086–1094. doi: 10.1128/jb.151.3.1086-1094.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed D. W., Clayton R. K. Isolation of a reaction center fraction from Rhodopseudomonas spheroides. Biochem Biophys Res Commun. 1968 Mar 12;30(5):471–475. doi: 10.1016/0006-291x(68)90075-2. [DOI] [PubMed] [Google Scholar]
- Reed D. W. Isolation and composition of a photosynthetic reaction center complex from Rhodopseudomonas spheroides. J Biol Chem. 1969 Sep 25;244(18):4936–4941. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Long S. R., Meade H. M., van den Bos R. C., Ausubel F. M. ISRm1: A Rhizobium meliloti insertion sequence that transposes preferentially into nitrogen fixation genes. J Mol Appl Genet. 1982;1(5):405–418. [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- SISTROM W. R. The kinetics of the synthesis of photopigments in Rhodopseudomonas spheroides. J Gen Microbiol. 1962 Sep;28:607–616. doi: 10.1099/00221287-28-4-607. [DOI] [PubMed] [Google Scholar]
- Sauer K., Austin L. A. Bacteriochlorophyll-protein complexes from the light-harvesting antenna of photosynthetic bacteria. Biochemistry. 1978 May 16;17(10):2011–2019. doi: 10.1021/bi00603a033. [DOI] [PubMed] [Google Scholar]
- Saunders V. A., Saunders J. R., Bennett P. M. Extrachromosomal deoxyribonucleic acid in wild-type and photosynthetically incompetent strains of Rhodopseudomonas spheroides. J Bacteriol. 1976 Mar;125(3):1180–1187. doi: 10.1128/jb.125.3.1180-1187.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Zieg J., Hilmen M., Simon M. Phase variation in Salmonella: genetic analysis of a recombinational switch. Proc Natl Acad Sci U S A. 1979 Jan;76(1):391–395. doi: 10.1073/pnas.76.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suyama Y., Gibson J. Satellite DNA in photosynthetic bacteria. Biochem Biophys Res Commun. 1966 Aug 23;24(4):549–553. doi: 10.1016/0006-291x(66)90355-x. [DOI] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]
- Zieg J., Silverman M., Hilmen M., Simon M. Recombinational switch for gene expression. Science. 1977 Apr 8;196(4286):170–172. doi: 10.1126/science.322276. [DOI] [PubMed] [Google Scholar]