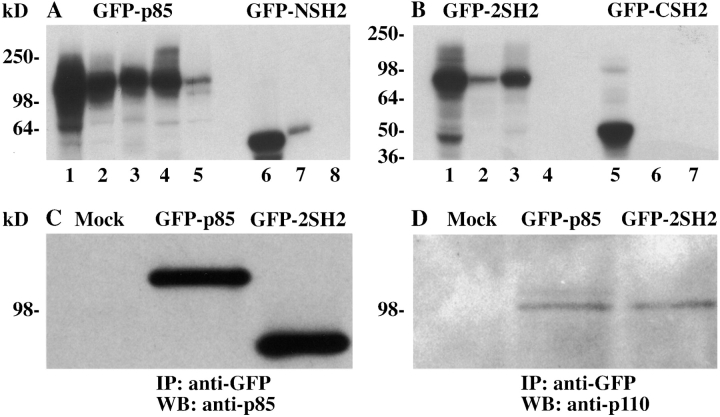
Figure 1.
Characterization of the GFP-p85 fusion constructs. The cDNA constructs were in vitro translated using [35S]methionine. GFP-p85 was immunoprecipitated with an antibody to GFP, the inter–NSH2-SH3 domain (U1), the SH3 domain (U13), the COOH-terminal SH2 domain (Transduction Laboratories), and with protein A–Sepharose alone (A, lanes 1–5, respectively). GFP-2SH2 was immunoprecipitated with antibodies to GFP, the NH2 (U14) and COOH termini, (Transduction Laboratories), and protein A–Sepharose alone (B, lanes 1–4, respectively). GFP-CSH2 and GFP-NSH2 were immunoprecipitated with anti-GFP, COOH- and NH2-terminal antibodies, respectively, and with a protein A–Sepharose control (B, lanes 5–7; A, lanes 6–8). To assess whether GFP-p85 bound to the p110 catalytic subunit, Cos-7 cells were transiently transfected with the GFP-p85, GFP-2SH2, and pcDNA3.1/Zeo constructs (Mock, C and D). The cells were lysed and immunoprecipitated with an antibody to GFP, transferred to nitrocellulose, probed with an antibody to p85 (C), and stripped and reprobed with an antibody to the p110 catalytic subunit of PI 3-kinase (D).