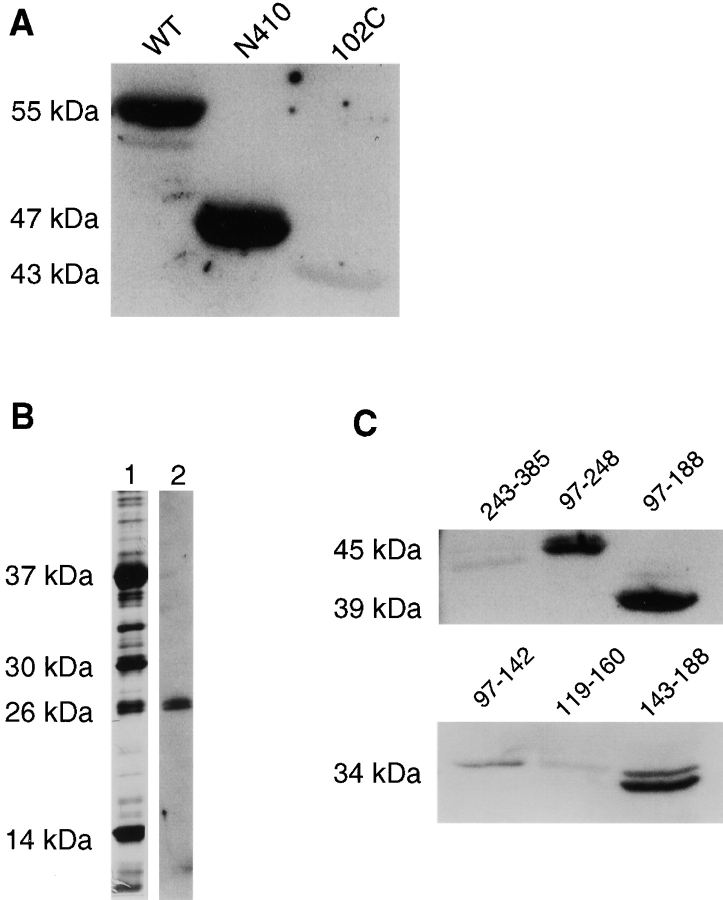
Figure 4.
CH1 domain of fimbrin binds the NH2 terminus of nonassembled vimentin. (A) In vitro expressed and purified wild type (WT), COOH-terminally deleted (N410) or NH2-terminally deleted (102C) vimentin were resolved on SDS-PAGE, transferred to PVDF, and probed with labeled fimbrin. Fimbrin binds wild type (WT) and the COOH-terminally deleted vimentin (N410) but not the NH2-terminal deleted vimentin (102C). (B) Limited proteolysis of fimbrin with Lys-C and the fragments were detected with silver stain (lane 1), electroblotted, and probed with biotinylated vimentin (lane 2). Vimentin bound a 26-kD fragment. (C) Overlays of fimbrin deletion constructs using biotinylated vimentin. Vimentin bound bands that contained residues 143–188 in the CH1 domain.