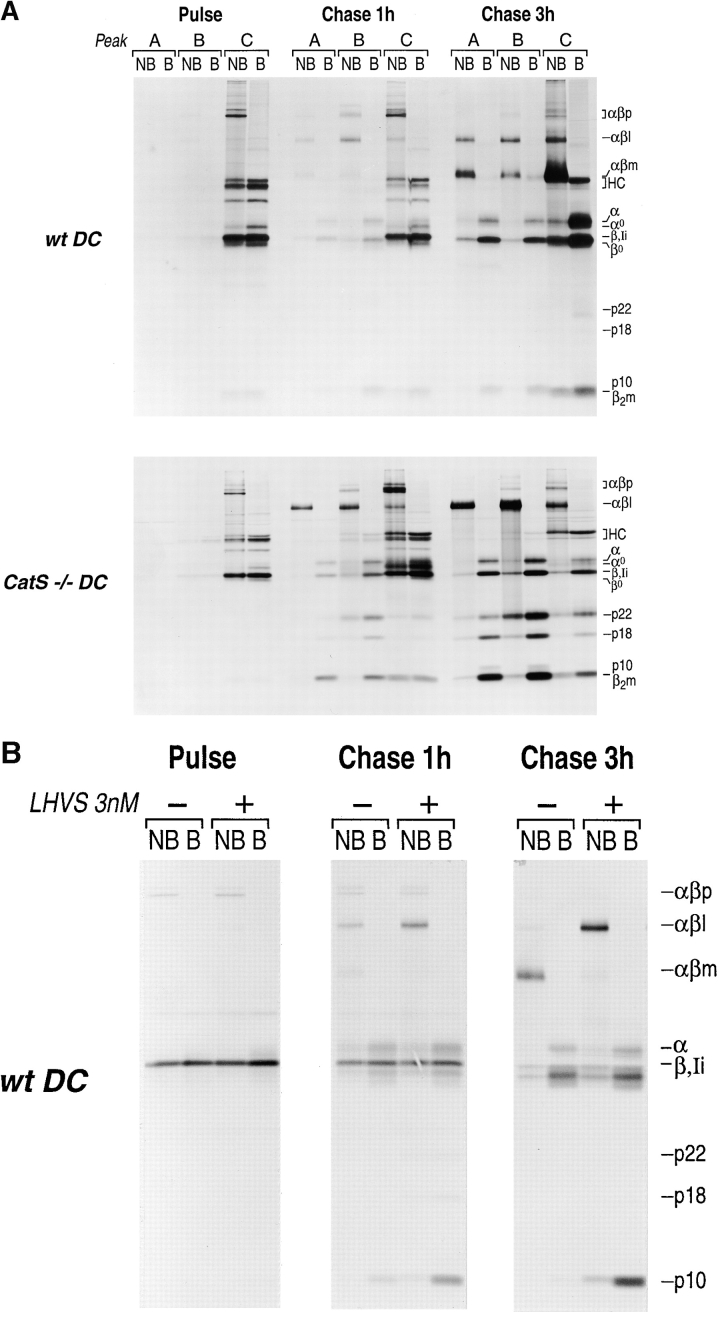
Figure 6.
(A) Subcellular distribution of MHC class II molecules in wt and CatS−/− DC under pulse chase-labeling conditions. Flt3-induced DC from wt (upper panel) and CatS−/− (lower panel) mice were metabolically labeled with [35S]methionine/cysteine for 30 min (pulse) and chased for 1 and 3 h. At each timepoint, subcellular fractions A (lysosomes), B (late endosomes), and C (early endosomes/PM and ER–Golgi) were generated as described. Immunoprecipitation from these fractions was performed with N22 (class II) and p8 (class I) at the same time. The samples were divided into two and analyzed by 12.5% SDS-PAGE either without (NB) or with prior boiling (B). (αβp, high molecular weight nonameric [αβIi]3 complexes; αβl, 70-kD SDS-stable complex consisting of αβ bound to the Ii degradation intermediates Ii-p10, Ii-p18, or Ii-p22; αβm, mature SDS-resistant αβ dimer bound to either CLIP or peptide; HC, MHC class I heavy chain; α, mature class II alpha chain; α0, immature α chain; β, mature β-chain; Ii, full-length invariant chain; β0, immature β-chain; p10/p18/p22, COOH-terminal degradation intermediates of Ii; β2m, β-2 microglobulin). (B) Effect of LHVS on the degradation of Ii in mature wt DC. DC were metabolically labeled for 30 min, and chased for 0, 1, or 3 h as described, either in the presence (+) or the absence (–) of the CatS inhibitor LHVS at 3 nM concentration. Cell lysates were prepared without any further subcellular fractionation steps and directly analyzed by immunoprecipitation using the N22 antibody. After separation by 12.5% SDS-PAGE with (B) or without prior boiling of the samples (NB), the samples were visualized by autoradiography.