Figure 5.
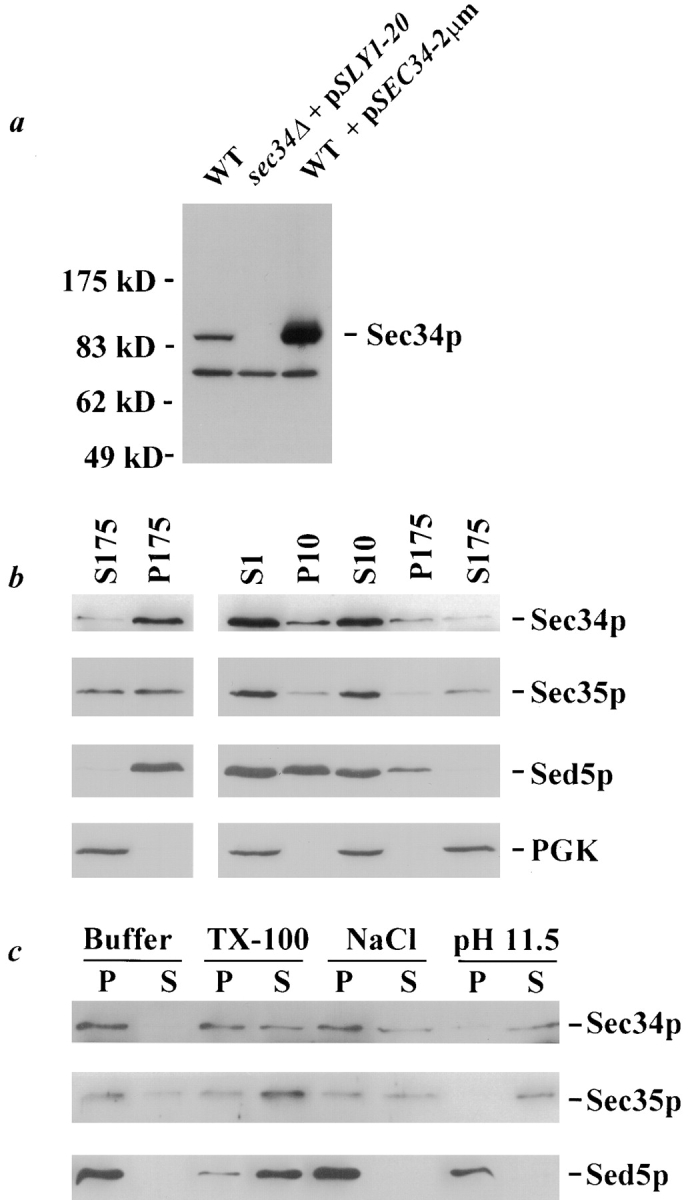
Biochemical characterization of Sec34p. a, Characterization of the anti-Sec34p antibody. Affinity-purified polyclonal antibodies against Sec34p were used to probe yeast lysate (derived from 0.2 OD units of cells per lane) from either a wild-type strain (RSY255), the sec34Δ strain expressing SLY1-20 to assist growth (GWY132), or a wild-type strain overexpressing SEC34 from a multicopy plasmid (RSY255 containing pSV25). Lysates were separated by SDS-7%PAGE and immunoblotted with affinity-purified antibody against Sec34p. The migration of molecular weight markers is indicated on the left. b, Subcellular fractionation of Sec34p. Left, Lysate (S1) from a wild-type strain (RSY255) was centrifuged at 175,000 g and separated into pellet (P) and supernatant (S) fractions. Right, S1 lysate was centrifuged at 10,000 g and separated into P10 and S10 fractions, and the S10 was further centrifuged at 175,000 g and separated into P175 and S175 fractions. c, Sec34p encodes a peripheral membrane protein. Isolated yeast membranes from a wild-type strain (RSY255) were incubated with buffer, 1% Triton X-100, 1 M NaCl, or 0.1 M Na2CO3, pH 11.5, as indicated, before centrifugation at 175,000 g and separation into pellet (P) and supernatant (S) fractions. b and c, Equivalent amounts (relative to the starting material) of each fraction were separated by SDS-12%PAGE and analyzed by immunoblotting with affinity-purified antibodies against Sec34p (only the upper, Sec34p-specific band is shown), Sec35p, Sed5p, and PGK, as indicated.
