Figure 8.
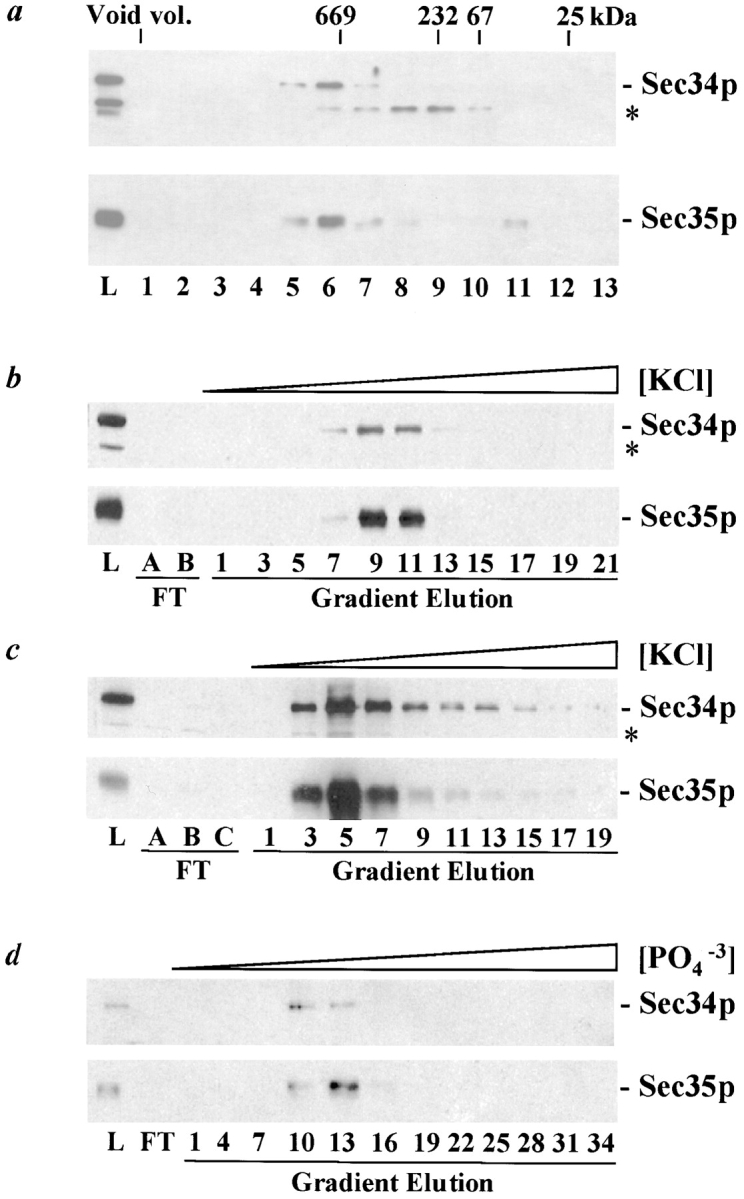
Sec34p and Sec35p are components of a large protein complex. a, Sec34p and Sec35p cofractionate by Superose 6 size-exclusion chromatography. Pooled fractions from a DEAE anion exchange column containing Sec34p and Sec35p were fractionated by size-exclusion chromatography. Proteins contained in the load (L), as well as the column, fractions were separated by SDS-11%PAGE and immunoblotted with antibodies against Sec34p or Sec35p, as indicated. The immunoreactive protein marked with an asterisk (*) is unrelated to Sec34p (see Fig. 5 a). The elution position of 25, 67, 232, and 669 kD protein standards, as well as the location of the void volume, is indicated at the top. b–d, Sec34p and Sec35p cofractionate through sequential chromatographic steps. b, Sec34p and Sec35p cofractionate by MonoQ anion exchange chromatography. The remainder of the DEAE anion exchange pool containing Sec34p and Sec35p was subjected to Sephacryl S-300 gel filtration chromatography, and the pooled Sec34p- and Sec35p-containing fractions were loaded onto a MonoQ anion exchange column and eluted with a linear KCl gradient. Proteins contained in the load (L), flow-through fractions (FT), and every second gradient elution fraction were analyzed as described in a. c, Sec34p and Sec35p cofractionate by MonoS cation exchange chromatography. Fractions 8–11 from the MonoQ step described in b were loaded onto a MonoS anion exchange column and eluted with a linear KCl gradient. Proteins contained in the load (L), flow-through fractions (FT), and every second gradient elution fraction were analyzed as described in a. d, Sec34p and Sec35p cofractionate on ceramic hydroxyapatite. Fractions 4–7 from the MonoS chromatographic step described in c were subjected to Superose 6 gel filtration chromatography and the Sec34p- and Sec35-containing fractions were pooled, loaded onto a ceramic hydroxyapatite column, and eluted with a linear potassium phosphate (PO4 −3) gradient. Proteins in the load (L), flow-through (FT), and every third gradient elution fraction were analyzed as described in a. The apparent difference in Sec34p and Sec35p abundance in fractions 10 and 13 is likely an artifact of chemiluminescent detection because reanalysis of every fraction in this region yields precise comigration and relative abundance (data not shown).
