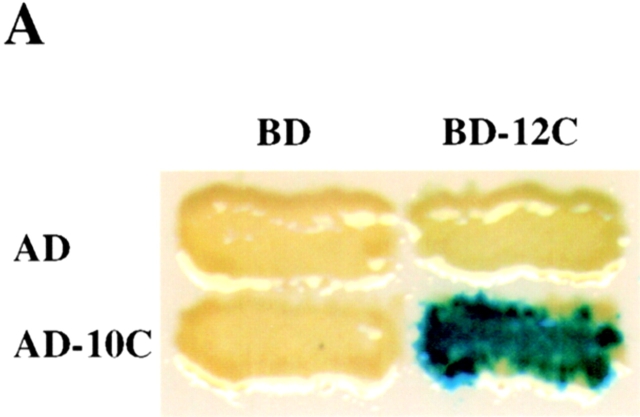
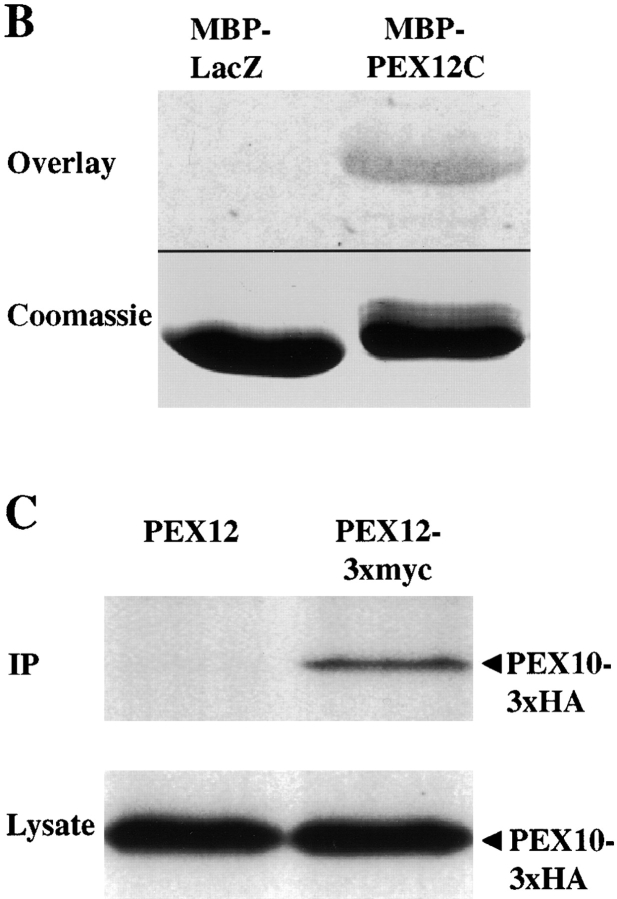
Figure 3.
The COOH-terminal domain of PEX12 interacts with the COOH-terminal domain of PEX10 in the yeast two-hybrid system. Two-hybrid reporter strains expressing the indicated fusion proteins were transferred to a nitrocellulose filter, submerged in liquid nitrogen to lyse the cells, and assayed for β-galactosidase activity. AD, GAL4 activation domain; and BD, GAL4-binding domain. (B) PEX10 binds immobilized PEX12C in vitro. 10 μg of purified MBP-LacZα and purified MBP-PEX12C were resolved by SDS-PAGE, transferred to membranes, and probed with 35S-labeled PEX10 (upper panel). A duplicate gel was stained before transfer with Coomassie blue (lower panel). (C) Coimmunoprecipitation of PEX10 with PEX12. Lysates from cells cotransfected with either pcDNA3-PEX12 and pcDNA3-PEX10/3xHA (left lane) or pcDNA3-PEX12/3xmyc and pcDNA3-PEX10/3xHA (right lane) were immunoprecipitated with anti–myc antibodies and analyzed by immunoblot with anti–HA antibodies. Aliquots of the crude lysates before immunoprecipitation were also assayed for PEX10/3xHA levels by immunoblot.