Abstract
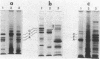
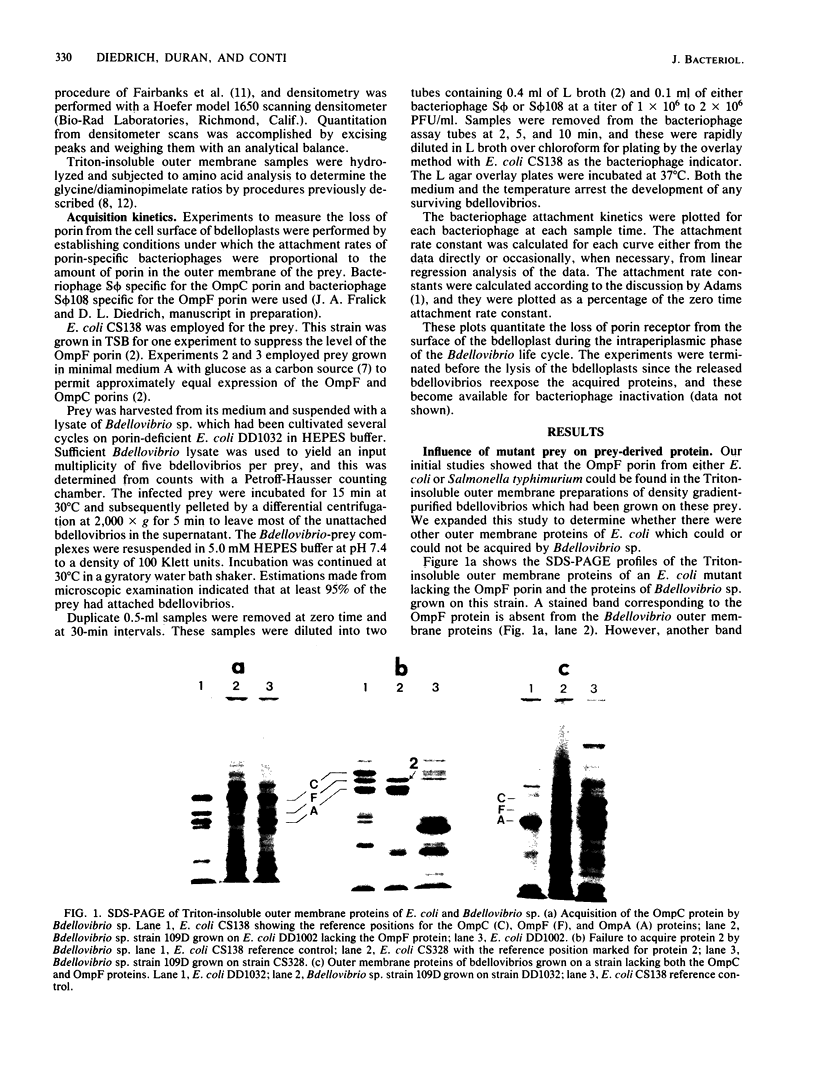
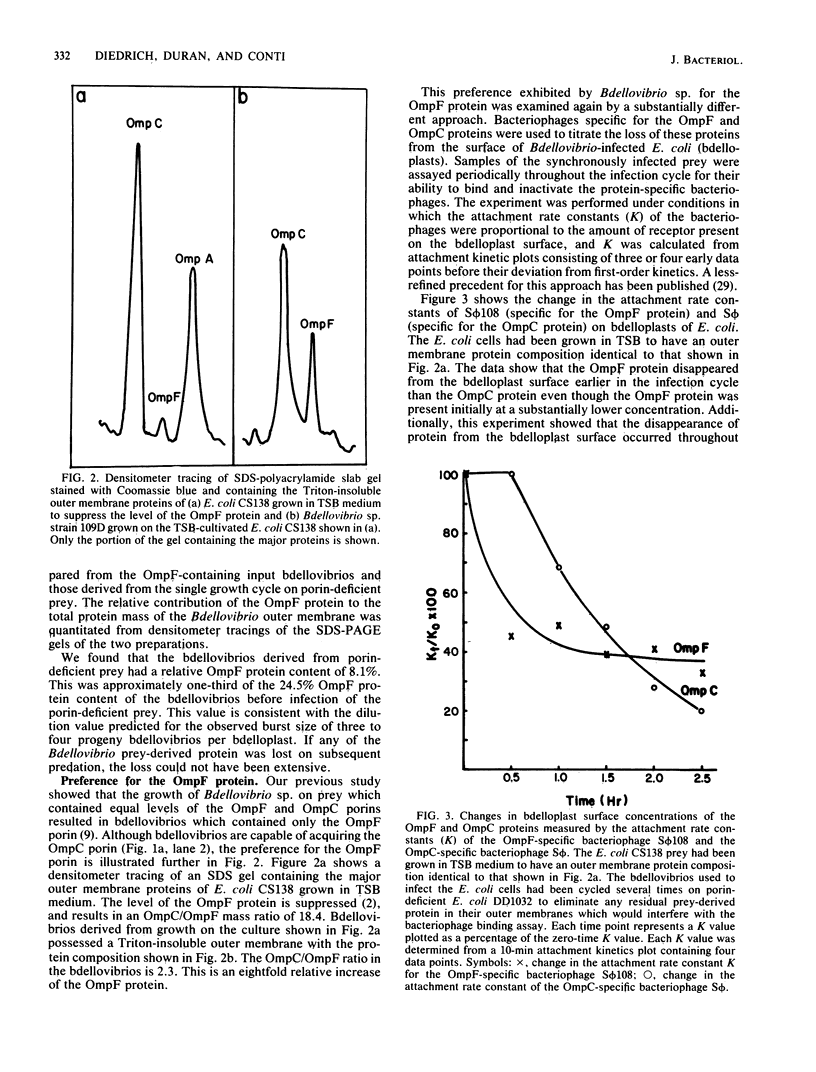
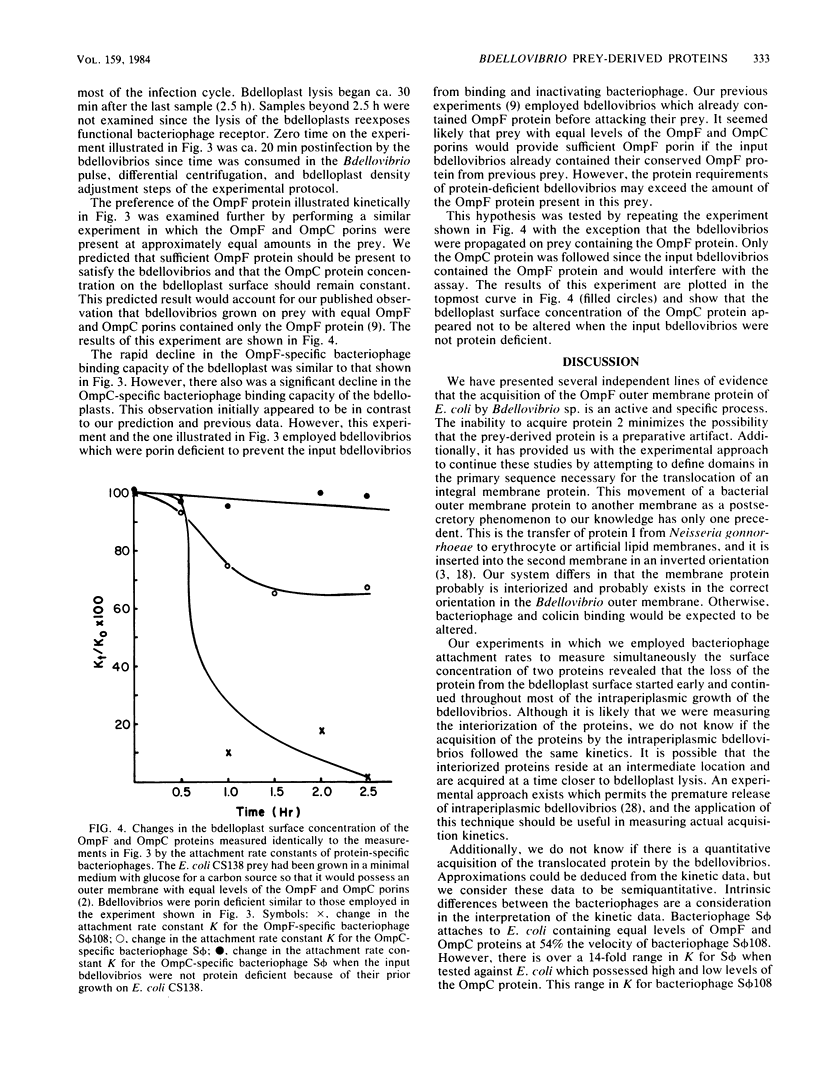
The ability of Bdellovibrio sp. to acquire the OmpF major outer membrane protein from its Escherichia coli prey was examined to determine if there were other outer membrane proteins which could or could not be acquired. Growth of bdellovibrios on mutant prey which were defective in the expression of outer membrane proteins revealed that Bdellovibrio sp. could acquire the OmpC protein in the absence of the OmpF protein. However, the OmpA, LamB, and protein 2 proteins could not be found in the Bdellovibrio Triton-insoluble outer membrane. The disappearance of the OmpF and OmpC proteins from the bdelloplast surface was measured, and it was determined that Bdellovibrio sp. exhibited a kinetic and temporal preference for the OmpF protein. Bdellovibrios could be grown on porin-deficient prey, and the progeny bdellovibrios possessed outer membranes with a protein mass deficiency.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassford P. J., Jr, Diedrich D. L., Schnaitman C. L., Reeves P. Outer membrane proteins of Escherichia coli. VI. Protein alteration in bacteriophage-resistant mutants. J Bacteriol. 1977 Aug;131(2):608–622. doi: 10.1128/jb.131.2.608-622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C. Gonococcal membrane proteins: speculation on their role in pathogenesis. Prog Allergy. 1983;33:298–313. [PubMed] [Google Scholar]
- Braun V., Krieger-Brauer H. J. Interrelationship of the phage lambda receptor protein and maltose transport in mutants of Escherichia coli K12. Biochim Biophys Acta. 1977 Aug 15;469(1):89–98. doi: 10.1016/0005-2736(77)90328-5. [DOI] [PubMed] [Google Scholar]
- Chai T., Wu V., Foulds J. Colicin A receptor: role of two Escherichia coli outer membrane proteins (OmpF protein and btuB gene product) and lipopolysaccharide. J Bacteriol. 1982 Aug;151(2):983–988. doi: 10.1128/jb.151.2.983-988.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich D. L., Fralick J. A. Relationship between the OmpC and LamB proteins of Escherichia coli and its influence on the protein mass of the outer membrane. J Bacteriol. 1982 Jan;149(1):156–160. doi: 10.1128/jb.149.1.156-160.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich D. L., Summers A. O., Schnaitman C. A. Outer membrane proteins of Escherichia coli. V. Evidence that protein 1 and bacteriophage-directed protein 2 are different polypeptides. J Bacteriol. 1977 Aug;131(2):598–607. doi: 10.1128/jb.131.2.598-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fralick J. A., Diedrich D. L. Studies on the expression of outer membrane protein 2 in escherichia coli. Mol Gen Genet. 1982;188(1):139–142. doi: 10.1007/BF00333008. [DOI] [PubMed] [Google Scholar]
- Gabay J., Yasunaka K. Interaction of the lamB protein with the peptidoglycan layer in Escherichia coli K12. Eur J Biochem. 1980 Feb;104(1):13–18. doi: 10.1111/j.1432-1033.1980.tb04393.x. [DOI] [PubMed] [Google Scholar]
- Hindennach I., Henning U. The major proteins of the Excherichia coli outer cell envelope membrane. Preparative isolation of all major membrane proteins. Eur J Biochem. 1975 Nov 1;59(1):207–213. doi: 10.1111/j.1432-1033.1975.tb02443.x. [DOI] [PubMed] [Google Scholar]
- Kuenen J. G., Rittenberg S. C. Incorporation of long-chain fatty acids of the substrate organism by Bdellovibrio bacteriovorus during intraperiplasmic growth. J Bacteriol. 1975 Mar;121(3):1145–1157. doi: 10.1128/jb.121.3.1145-1157.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Diffusion of solutes through channels produced by phage lambda receptor protein of Escherichia coli: inhibition by higher oligosaccharides of maltose series. Biochem Biophys Res Commun. 1980 Mar 13;93(1):166–171. doi: 10.1016/s0006-291x(80)80261-0. [DOI] [PubMed] [Google Scholar]
- Lynch E. C., Blake M. S., Gotschlich E. C., Mauro A. Studies of Porins: Spontaneously Transferred from Whole Cells and Reconstituted from Purified Proteins of Neisseria gonorrhoeae and Neisseria meningitidis. Biophys J. 1984 Jan;45(1):104–107. doi: 10.1016/S0006-3495(84)84127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Rittenberg S. C. Incorporation of substrate cell lipid A components into the lipopolysaccharide of intraperiplasmically grown Bdellovibrio bacteriovorus. J Bacteriol. 1981 Sep;147(3):860–868. doi: 10.1128/jb.147.3.860-868.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Westermann P. Arrangement of the maltose-inducible major outer membrane proteins, the bacteriophage lambda receptor in Escherichia coli and the 44 K protein in Salmonella typhimurium. FEBS Lett. 1979 Mar 1;99(1):77–80. doi: 10.1016/0014-5793(79)80253-7. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Factors affecting the electrophoretic mobility of the major outer membrane proteins of Escherichia coli in polyacrylamide gels. Biochim Biophys Acta. 1979 Nov 23;581(1):163–178. doi: 10.1016/0005-2795(79)90233-2. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Identification of three genes controlling production of new outer membrane pore proteins in Escherichia coli K-12. J Bacteriol. 1978 Sep;135(3):1118–1129. doi: 10.1128/jb.135.3.1118-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Outer membrane proteins of Escherichia coli. VII. Evidence that bacteriophage-directed protein 2 functions as a pore. J Bacteriol. 1978 Mar;133(3):1181–1189. doi: 10.1128/jb.133.3.1181-1189.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg S. C. Nonidentity of Bdellovibrio bacteriovorus strains 109D and 109J. J Bacteriol. 1972 Jan;109(1):432–433. doi: 10.1128/jb.109.1.432-433.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Ruby E. G., Rittenberg S. C. Differentiation after premature release of intraperiplasmically growing Bdellovibrio bacteriovorous. J Bacteriol. 1983 Apr;154(1):32–40. doi: 10.1128/jb.154.1.32-40.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOLP H., STARR M. P. BDELLOVIBRIO BACTERIOVORUS GEN. ET SP. N., A PREDATORY, ECTOPARASITIC, AND BACTERIOLYTIC MICROORGANISM. Antonie Van Leeuwenhoek. 1963;29:217–248. doi: 10.1007/BF02046064. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Smith D., de Salsas M. F. Temperate Bacteriophage Which Causes the Production of a New Major Outer Membrane Protein by Escherichia coli. J Virol. 1975 May;15(5):1121–1130. doi: 10.1128/jvi.15.5.1121-1130.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr M. P., Huang J. C. Physiology of the bdellovibrios. Adv Microb Physiol. 1972;8:215–261. doi: 10.1016/s0065-2911(08)60191-5. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Rittenberg S. C. Penicillin-induced formation of osmotically stable spheroplasts in nongrowing Bdellovibrio bacteriovorus. J Bacteriol. 1978 Mar;133(3):1484–1491. doi: 10.1128/jb.133.3.1484-1491.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Nogami T., Mizushima S. Arrangement of bacteriophage lambda receptor protein (LamB) in the cell surface of Escherichia coli: a reconstitution study. J Bacteriol. 1981 Aug;147(2):660–669. doi: 10.1128/jb.147.2.660-669.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]