Abstract
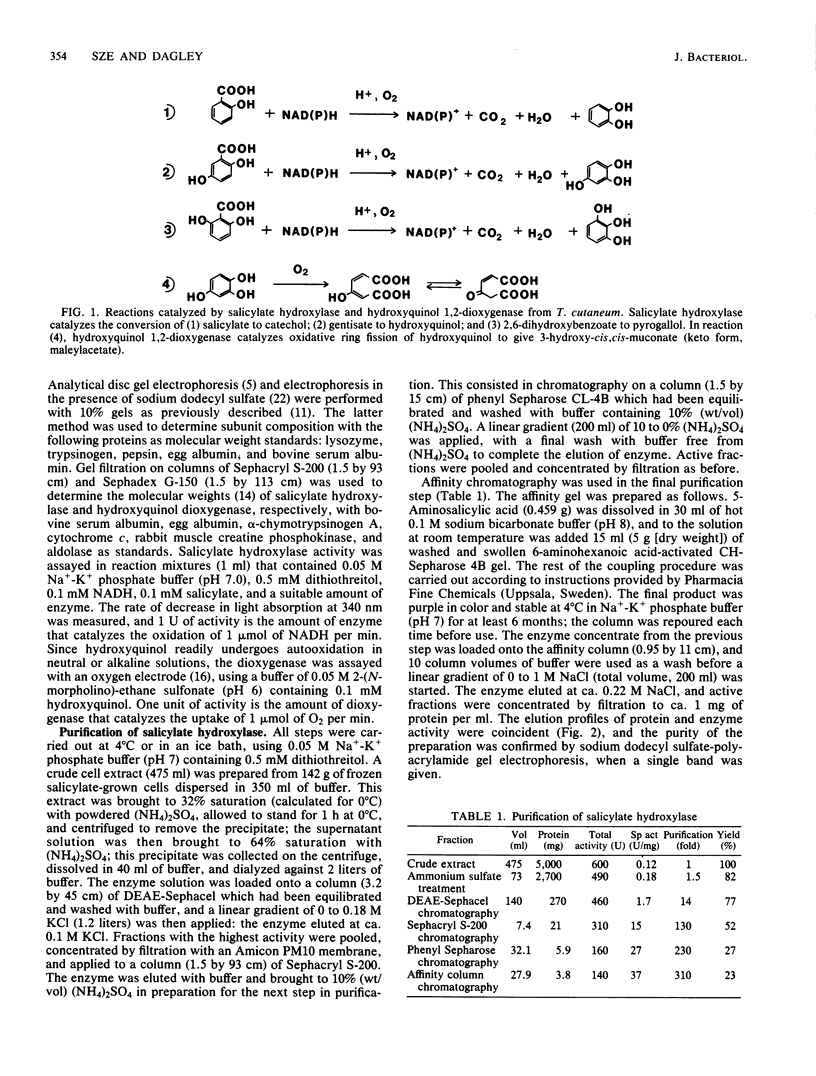
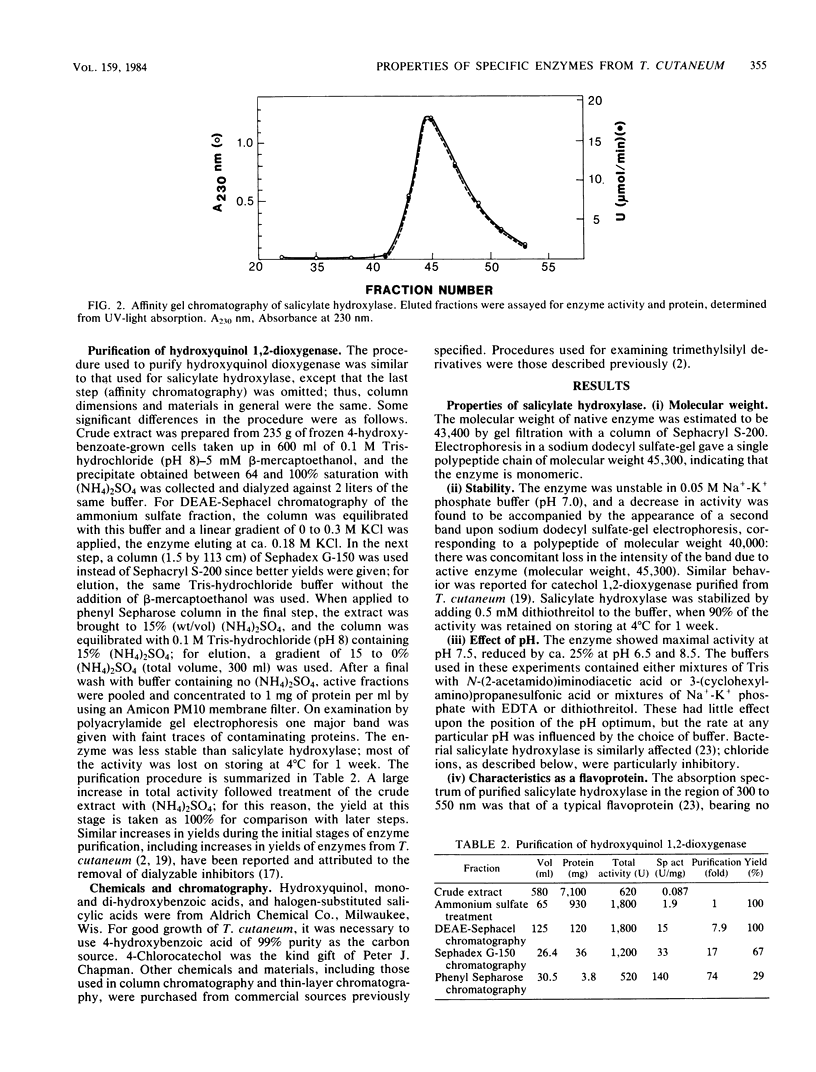
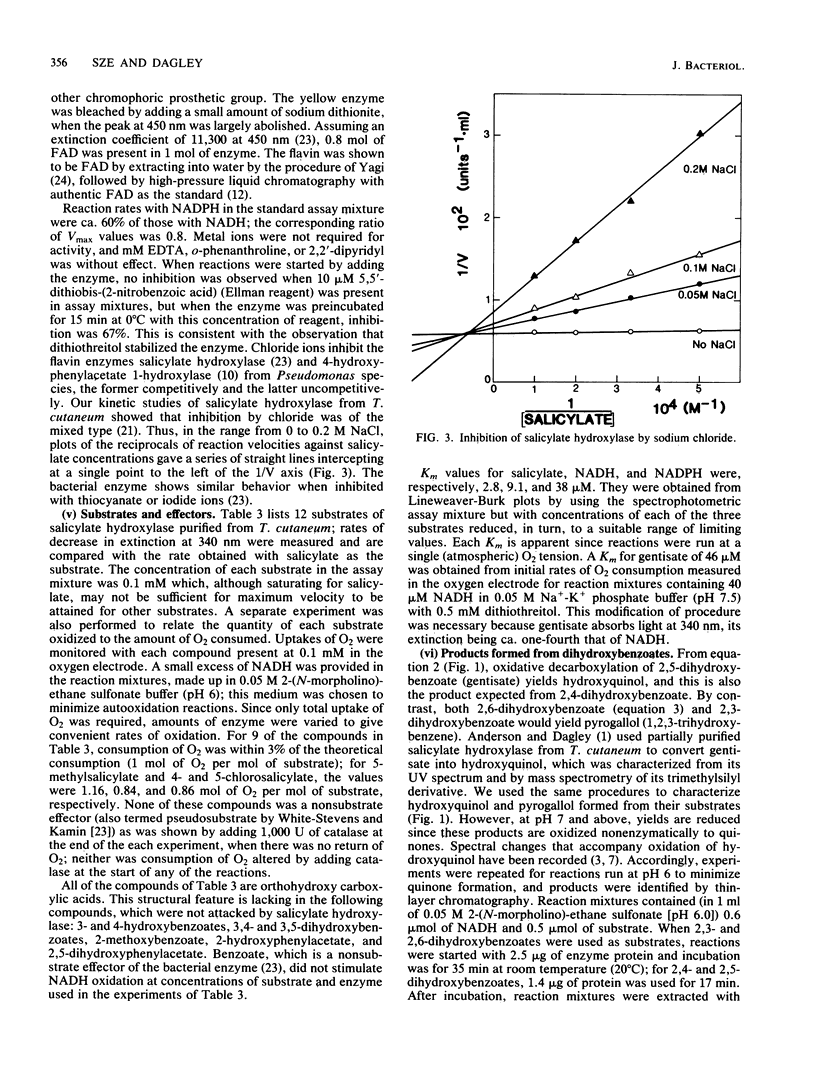
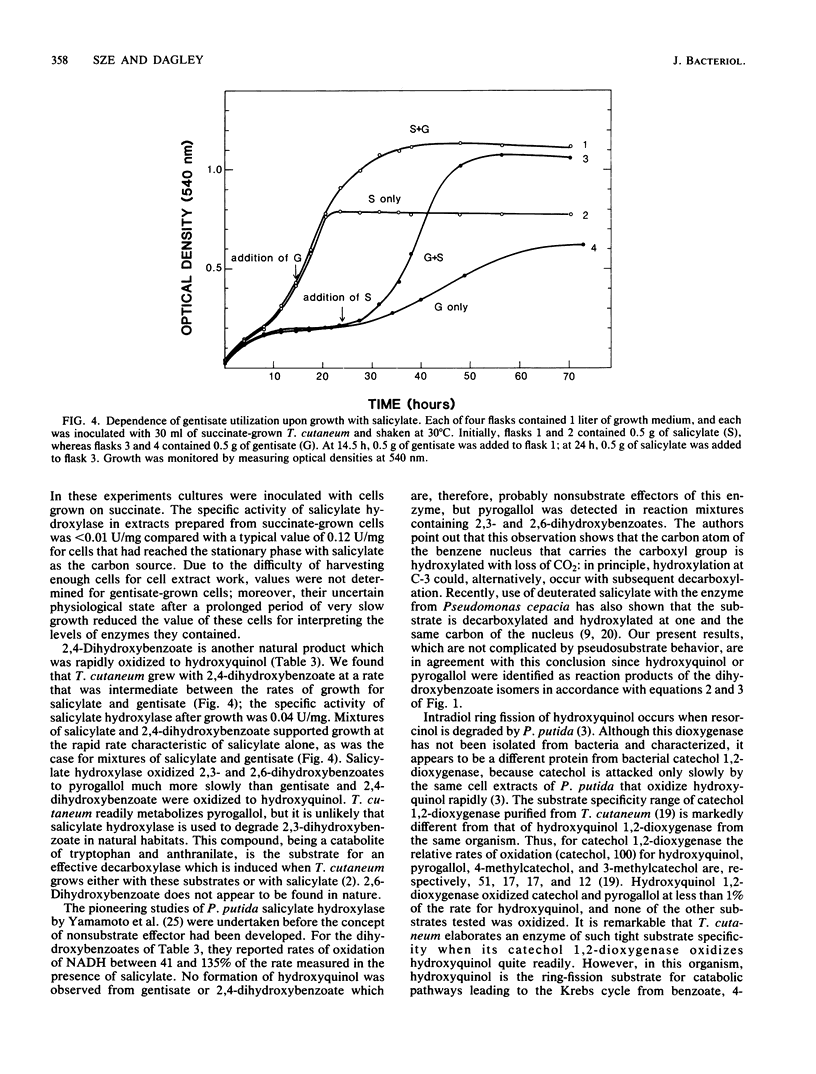
Salicylate hydroxylase (salicylate 1-monooxygenase, EC 1.14.13.1) was purified from the soil yeast Trichosporon cutaneum. The enzyme contained flavin adenine dinucleotide and was monomeric, with a molecular weight of 45,300. In addition to salicylate, the four isomeric dihydroxybenzoates having one hydroxyl adjacent to carboxyl in the benzene nucleus were oxidatively decarboxylated without formation of hydrogen peroxide. One of these isomers, gentisate, was rapidly oxidized to hydroxyquinol by the enzyme but did not serve as an effective single carbon source for T. cutaneum; however, when growing with salicylate, cells also readily utilized gentisate for growth. Hydroxyquinol 1,2-dioxygenase (EC 1.13.11....) is a newly investigated enzyme which was purified from T. cutaneum grown with 4-hydroxybenzoate. The enzyme was red, contained ferric iron, and was specific for hydroxyquinol; catechol and pyrogallol were oxidized at less than 1% of the rate for hydroxyquinol, and no activity could be detected against seven other catechols. The enzyme was composed of two nonidentical subunits having molecular weights of 39,600 and 38,200 and was apparently dimeric.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. J., Dagley S. Catabolism of aromatic acids in Trichosporon cutaneum. J Bacteriol. 1980 Feb;141(2):534–543. doi: 10.1128/jb.141.2.534-543.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. J., Dagley S. Catabolism of tryptophan, anthranilate, and 2,3-dihydroxybenzoate in Trichosporon cutaneum. J Bacteriol. 1981 Apr;146(1):291–297. doi: 10.1128/jb.146.1.291-297.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman P. J., Ribbons D. W. Metabolism of resorcinylic compounds by bacteria: alternative pathways for resorcinol catabolism in Pseudomonas putida. J Bacteriol. 1976 Mar;125(3):985–998. doi: 10.1128/jb.125.3.985-998.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Gaal A. B., Neujahr H. Y. Maleylacetate reductase from Trichosporon cutaneum. Biochem J. 1980 Mar 1;185(3):783–786. doi: 10.1042/bj1850783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal A., Neujahr H. Y. Metabolism of phenol and resorcinol in Trichosporon cutaneum. J Bacteriol. 1979 Jan;137(1):13–21. doi: 10.1128/jb.137.1.13-21.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzah R. Y., Tu S. C. Determination of the position of monooxygenation in the formation of catechol catalyzed by salicylate hydroxylase. J Biol Chem. 1981 Jun 25;256(12):6392–6394. [PubMed] [Google Scholar]
- Hareland W. A., Crawford R. L., Chapman P. J., Dagley S. Metabolic function and properties of 4-hydroxyphenylacetic acid 1-hydroxylase from Pseudomonas acidovorans. J Bacteriol. 1975 Jan;121(1):272–285. doi: 10.1128/jb.121.1.272-285.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung P. T., Chapman P. J., Dagley S. Purification and properties of 4-hydroxy-2-ketopimelate aldolase from Acinetobacter. J Bacteriol. 1974 Oct;120(1):168–172. doi: 10.1128/jb.120.1.168-172.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light D. R., Walsh C., Marletta M. A. Analytical and preparative high-performance liquid chromatography separation of flavin and flavin analog coenzymes. Anal Biochem. 1980 Nov 15;109(1):87–93. doi: 10.1016/0003-2697(80)90014-7. [DOI] [PubMed] [Google Scholar]
- Nakai C., Kagamiyama H., Saeki Y., Nozaki M. Nonidentical subunits of pyrocatechase from Pseudomonas arvilla C-1. Arch Biochem Biophys. 1979 Jun;195(1):12–22. doi: 10.1016/0003-9861(79)90322-9. [DOI] [PubMed] [Google Scholar]
- Rao P. V., Moore K., Towers G. H. O-pyrocatechiuc acid carboxy-lyase from Aspergillus niger. Arch Biochem Biophys. 1967 Nov;122(2):466–473. doi: 10.1016/0003-9861(67)90220-2. [DOI] [PubMed] [Google Scholar]
- Sparnins V. L., Burbee D. G., Dagley S. Catabolism of L-tyrosine in Trichosporon cutaneum. J Bacteriol. 1979 May;138(2):425–430. doi: 10.1128/jb.138.2.425-430.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J. M., Neujahr H. Y. Purification and properties of catechol 1,2-oxygenase from Trichosporon cutaneum. Eur J Biochem. 1970 Feb;12(3):427–434. doi: 10.1111/j.1432-1033.1970.tb00869.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- White-Stevens R. H., Kamin H. Studies of a flavoprotein, salicylate hydroxylase. I. Preparation, properties, and the uncoupling of oxygen reduction from hydroxylation. J Biol Chem. 1972 Apr 25;247(8):2358–2370. [PubMed] [Google Scholar]
- YAGI K. Chemical determination of flavins. Methods Biochem Anal. 1962;10:319–356. doi: 10.1002/9780470110270.ch10. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO S., KATAGIRI M., MAENO H., HAYAISHI O. SALICYLATE HYDROXYLASE, A MONOOXYGENASE REQUIRING FLAVIN ADENINE DINUCLEOTIDE. I. PURIFICATION AND GENERAL PROPERTIES. J Biol Chem. 1965 Aug;240:3408–3413. [PubMed] [Google Scholar]
- Yoshida R., Hori K., Fujiwara M., Saeki Y., Kagamiyama H. Nonidentical subunits of protocatechuate 3,4-dioxygenase. Biochemistry. 1976 Sep 7;15(18):4048–4053. doi: 10.1021/bi00663a020. [DOI] [PubMed] [Google Scholar]


