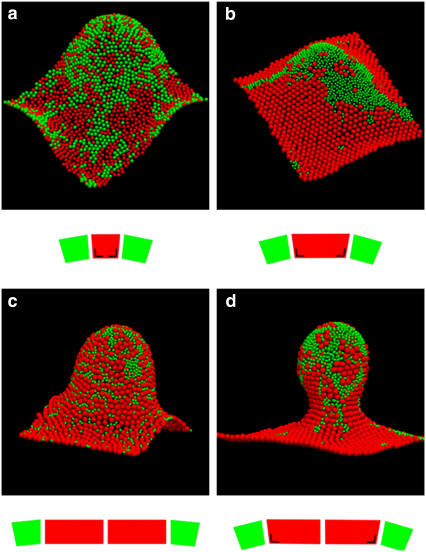
FIGURE 6.
Simulation snapshots of equilibrium configurations of the membrane with ∼1500 protein complexes, 50% of them being RC-LH1 (red) and 50% LH2 (green). Periodic boundary conditions ensure that the sides of the membrane in opposite directions match up. The snapshots are accompanied by schematic diagrams illustrating the size ratios and the spontaneous curvature of contacts between RC-LH1 (red) and LH2 (green) complexes. The angle mark drawn on the RC-LH1 truncated cone denotes LH2-induced deformation (see also Fig. 5). Domain formation is enhanced by the size difference of the complexes: (a) equal-size beads, (b) RC-LH1 monomers, and (c) RC-LH1 dimers. Larger crystalline domains of RC-LH1 appear when increasing the spontaneous curvature factor c0 RC-LH1-LH2/c0 LH2-LH2 = 1/3 (c) to 1 (a, b, and d). Local compositions couple to the local curvature of the membrane, with flat RC-LH1 regions and positively curved LH2 clusters. The area fraction occupied by the complexes is 0.71 in all simulations but the ones with equal size beads, for which it is 0.68. All simulations were started with a flat random configuration and run at constant c0 LH2-LH2 = 0.05 σ−1 (corresponds to a conical angle of ∼3° for the LH2), c0 RC-LH1-RC-LH1 = 0, k = 40 kT, and kA = 10 kTσ−2, where σ is the diameter of the LH2 complex. RC-LH1s have a diameter of σ in snapshot (a) and 2σ in the rest.