Abstract
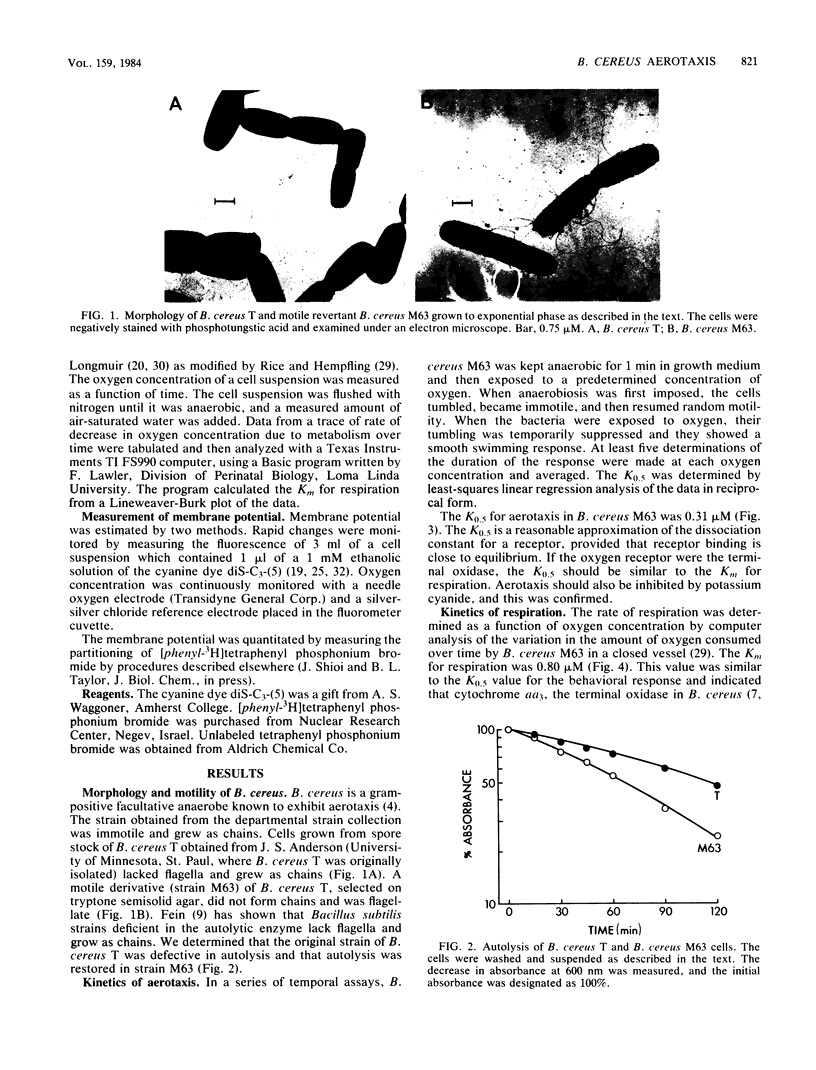
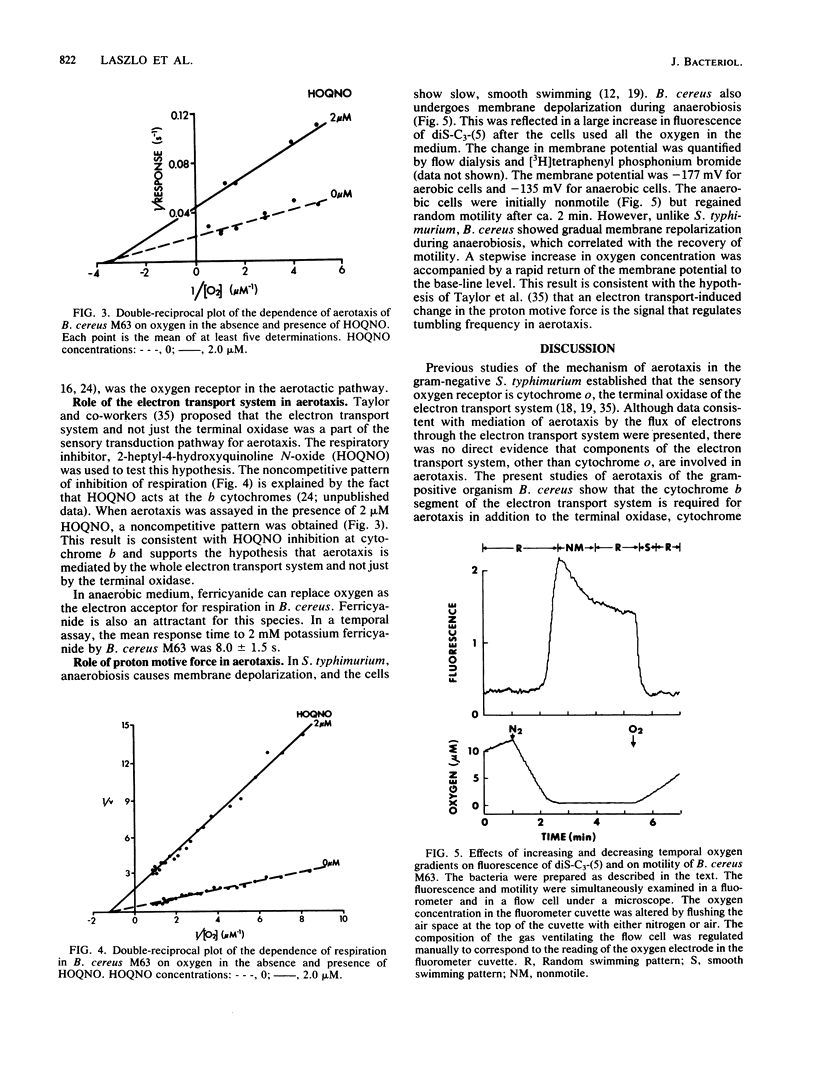
Aerotaxis (migration towards oxygen) of Bacillus cereus M63, a motile strain, was inhibited by potassium cyanide and 2-heptyl-4-hydroxyquinoline N-oxide, indicating a requirement for both the terminal oxidase (cytochrome aa3) and the cytochrome b segment of the electron transport system. The concentration of oxygen that gave a half-maximal aerotactic response (K0.5) was 0.31 microM, which was similar to the Km for respiration (0.80 microM). The proton motive force increased from -135 to -177 mV when anaerobic cells were aerated, and it is proposed that the signal for aerotaxis is the increase in proton motive force that results from increased respiration. A strain of B. cereus T initially used in this study was immotile, grew as long chains of cells, and was deficient in autolytic enzyme. B. cereus M63 is a spontaneous derivative of B. cereus T that has normal motility.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Science. 1966 Aug 12;153(3737):708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- Adler J., Epstein W. Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2895–2899. doi: 10.1073/pnas.71.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARACCHINI O., SHERRIS J. C. The chemotactic effect of oxygen on bacteria. J Pathol Bacteriol. 1959 Apr;77(2):565–574. doi: 10.1002/path.1700770228. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Boyd A., Simon M. Bacterial chemotaxis. Annu Rev Physiol. 1982;44:501–517. doi: 10.1146/annurev.ph.44.030182.002441. [DOI] [PubMed] [Google Scholar]
- DOI R. H., HALVORSON H. Comparison of electron transport systems in vegetative cells and spores of Bacillus cereus. J Bacteriol. 1961 Jan;81:51–58. doi: 10.1128/jb.81.1.51-58.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckmann M. M. Mutant of Bacillus subtilis with a temperature-sensitive autolytic amidase. J Bacteriol. 1973 May;114(2):798–803. doi: 10.1128/jb.114.2.798-803.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein J. E. Possible involvement of bacterial autolytic enzymes in flagellar morphogenesis. J Bacteriol. 1979 Feb;137(2):933–946. doi: 10.1128/jb.137.2.933-946.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goy M. F., Springer M. S., Adler J. Sensory transduction in Escherichia coli: role of a protein methylation reaction in sensory adaptation. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4964–4968. doi: 10.1073/pnas.74.11.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer G. L., Harayama S. Sensory transduction in bacterial chemotaxis. Int Rev Cytol. 1983;81:33–70. doi: 10.1016/s0074-7696(08)62334-7. [DOI] [PubMed] [Google Scholar]
- Khan S., Macnab R. M. The steady-state counterclockwise/clockwise ratio of bacterial flagellar motors is regulated by protonmotive force. J Mol Biol. 1980 Apr 15;138(3):563–597. doi: 10.1016/s0022-2836(80)80018-0. [DOI] [PubMed] [Google Scholar]
- Kihara M., Macnab R. M. Cytoplasmic pH mediates pH taxis and weak-acid repellent taxis of bacteria. J Bacteriol. 1981 Mar;145(3):1209–1221. doi: 10.1128/jb.145.3.1209-1221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh H., Ball C. B., Adler J. Identification of a methyl-accepting chemotaxis protein for the ribose and galactose chemoreceptors of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):260–264. doi: 10.1073/pnas.76.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr Biochemistry of sensing and adaptation in a simple bacterial system. Annu Rev Biochem. 1981;50:765–782. doi: 10.1146/annurev.bi.50.070181.004001. [DOI] [PubMed] [Google Scholar]
- LONGMUIR I. S. Respiration rate of bacteria as a function of oxygen concentration. Biochem J. 1954 May;57(1):81–87. doi: 10.1042/bj0570081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. R., Felix J., Lundgren D. G. Development of a membrane-bound resiratory system prior to and during sporulation in Bacillus cereus and its relationship to membrane structure. J Bacteriol. 1972 Jun;110(3):968–977. doi: 10.1128/jb.110.3.968-977.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S. H., Adler J., Gargus J. J., Hogg R. W. Chemomechanical coupling without ATP: the source of energy for motility and chemotaxis in bacteria. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1239–1243. doi: 10.1073/pnas.71.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo D. J., Fandrich B. L., Sivaram A., Chance B., Taylor B. L. Cytochrome o as a terminal oxidase and receptor for aerotaxis in Salmonella typhimurium. J Bacteriol. 1984 Aug;159(2):663–667. doi: 10.1128/jb.159.2.663-667.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo D. J., Taylor B. L. Aerotaxis in Salmonella typhimurium: role of electron transport. J Bacteriol. 1981 Feb;145(2):990–1001. doi: 10.1128/jb.145.2.990-1001.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Koshland D. E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M. Sensory reception in bacteria. Symp Soc Exp Biol. 1982;35:77–104. [PubMed] [Google Scholar]
- Maeda K., Imae Y. Thermosensory transduction in Escherichia coli: inhibition of the thermoresponse by L-serine. Proc Natl Acad Sci U S A. 1979 Jan;76(1):91–95. doi: 10.1073/pnas.76.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeters G. A., Wilson D. F., Strobel G. A. Cytochromes in a cyanide-resistant strain of Bacillus cereus. Can J Microbiol. 1970 Dec;16(12):1221–1226. doi: 10.1139/m70-205. [DOI] [PubMed] [Google Scholar]
- Miller J. B., Koshland D. E., Jr Sensory electrophysiology of bacteria: relationship of the membrane potential to motility and chemotaxis in Bacillus subtilis. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4752–4756. doi: 10.1073/pnas.74.11.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwano M., Taylor B. L. Novel sensory adaptation mechanism in bacterial chemotaxis to oxygen and phosphotransferase substrates. Proc Natl Acad Sci U S A. 1982 Jan;79(1):11–15. doi: 10.1073/pnas.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske D. R., Adler J. Change in intracellular pH of Escherichia coli mediates the chemotactic response to certain attractants and repellents. J Bacteriol. 1981 Mar;145(3):1196–1208. doi: 10.1128/jb.145.3.1196-1208.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. W., Hempfling W. P. Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol. 1978 Apr;134(1):115–124. doi: 10.1128/jb.134.1.115-124.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader W. P., Anderson J. S. Membrane-bound nucleotidase of Bacillus cereus. J Bacteriol. 1978 Feb;133(2):576–583. doi: 10.1128/jb.133.2.576-583.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Springer M. S., Goy M. F., Adler J. Sensory transduction in Escherichia coli: two complementary pathways of information processing that involve methylated proteins. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3312–3316. doi: 10.1073/pnas.74.8.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. L., Miller J. B., Warrick H. M., Koshland D. E., Jr Electron acceptor taxis and blue light effect on bacterial chemotaxis. J Bacteriol. 1979 Nov;140(2):567–573. doi: 10.1128/jb.140.2.567-573.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. L. Role of proton motive force in sensory transduction in bacteria. Annu Rev Microbiol. 1983;37:551–573. doi: 10.1146/annurev.mi.37.100183.003003. [DOI] [PubMed] [Google Scholar]




