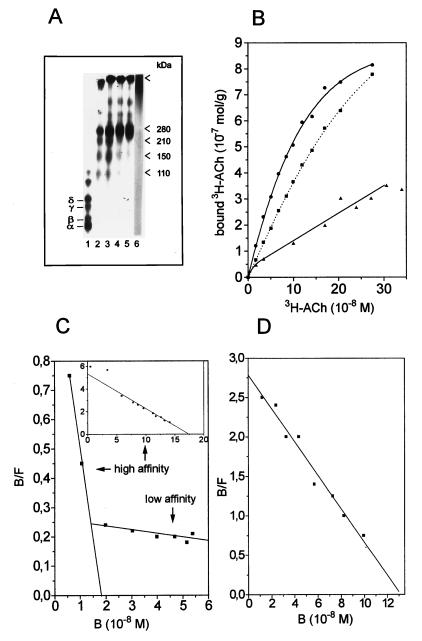
Figure 6.
Properties of the nAChR from Torpedo californica after cross-linking with the nonagonistic cross-linker glutardialdehyde. (A) Gel electrophoresis (4% acrylamide) of native nAChR (lane 1), Triton X-100-solubilized nAChR cross-linked with 0.04% glutardialdehyde for 1.5 h (lane 2), 3 h (lane 3), 8 h (lane 4), or 20 h (lane 5) and nAChR-rich membranes cross-linked with 0.04% glutardialdehyde for 20 h (lane 6). Arrows indicate subunit dimers (≈110 kDa), trimers (≈150 kDa), tetramers (≈210 kDa), and pentamers (≈280 kDa) and polymerized nAChR (from bottom to top). No intermediate stages of cross-linking can be seen after receptor cross-linking in membrane vesicles. (B) [3H]ACh binding after cross-linking with glutardialdehyde, in the absence (▴) and in the presence (•) of carbamoylcholine as compared with native nAChR (▪). Carbamoylcholine was removed before the [3H]ACh binding assay and the control experiment (native receptor plus carbamoylcholine) was performed under the same conditions. (C) Scatchard plot after cross-linking with glutardialdehyde in the absence of carbamoylcholine. A low-affinity binding site (Kd = −1/m = 2–5 μM; nH = 1.0) and a high-affinity binding site can be seen. (Inset) Scatchard plot of the high-affinity binding site alone after glutardialdehyde cross-linking, as measured by using a much higher amount of protein to achieve higher concentrations of bound [3H]ACh (Kd = 35 nM; nH = 1.0). The low-affinity binding site cannot be seen under these conditions. (D) Scatchard plot after cross-linking with glutardialdehyde in the presence of carbamoylcholine (Kd = 45 nM; nH = 1.0).