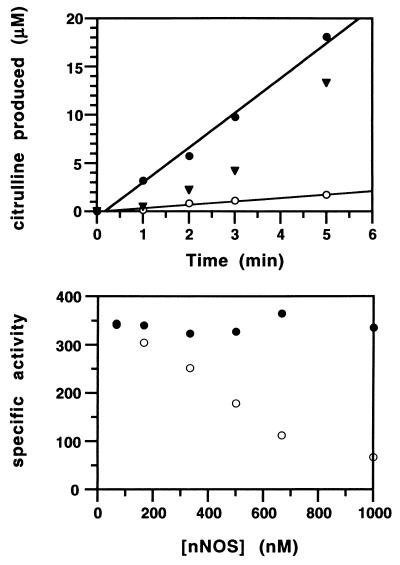
Figure 1.
nNOS catalysis in metal-depleted buffer. (Upper) Effect of transition metals on nNOS catalysis during typical assay conditions [≈30 nM nNOS, 37°C, 100 μM l-[14C]arginine (1.5 mCi/mmol), 10 μM H4B, 100 μM DTT, 1 mM CaCl2, 100 nM calmodulin, and 500 μM NADPH, pH 7.4]; after chelation treatment this mixture retained ≈100 nM nonheme iron. The assay volume was 300 μl. ▾, nNOS activity in the absence of exogenous transition metals, showing an initial lag in citrulline formation that is not observed when the reaction is carried out in the presence of 10 μM FeCl2 (•). ○, The inhibitory effect of 20 μM CuCl2 added to the assays. Assays were done in triplicate and plotted as the mean. Absolute values varied less than 5%. (Lower) Effect of increasing nNOS concentration (67 nM to 1.0 μM) on the specific activity (nmol⋅min−1⋅mg−1), in the presence (•) and absence (○) of 20 μM added FeCl2. The assays were carried out at 37°C for 2 min in the presence of 500 μM l-arginine. Assays were done in duplicate and plotted as the mean. Absolute values varied <5%.