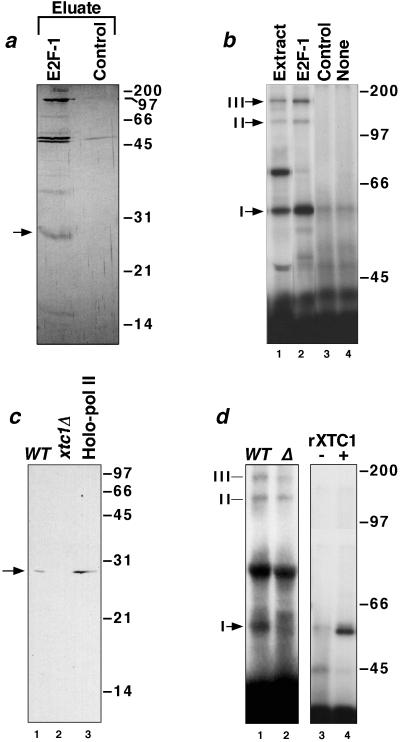
Figure 2.
Purification and cloning of Xtc1p. (a) Silver-stained SDS gel of the high-salt eluates from a LexA-E2F-1 (lane 1) and a control LexA (lane 2) affinity column. A 28-kDa yeast polypeptide that binds specifically to the E2F-1 activation domain is indicated. (b) Crosslinking by photoreactive LexA-E2F-1 in a yeast transcription extract (lane 1), in eluates from the LexA-E2F-1 and LexA affinity columns (lanes 2 and 3), or in the absence of added protein (lane 4). (c) Immunoblots with anti-Xtc1p of extracts (50 μg) prepared from isogenic wild-type (lane 1) and xtc1Δ (lane 2) yeast strains and of an aliquot (100 μg) of TFIIS affinity-purified yeast RNA pol II holoenzyme (lane 3) (27). (d) SDS-gel analysis of crosslinking by photoreactive LexA-E2F-1 to proteins in extracts from wild-type and xtc1Δ yeast cells (lanes 1 and 2) and crosslinking by photoreactive LexA-E2F-1 in the absence and presence of 200 ng of purified recombinant Xtc1p (lanes 3 and 4).