Abstract
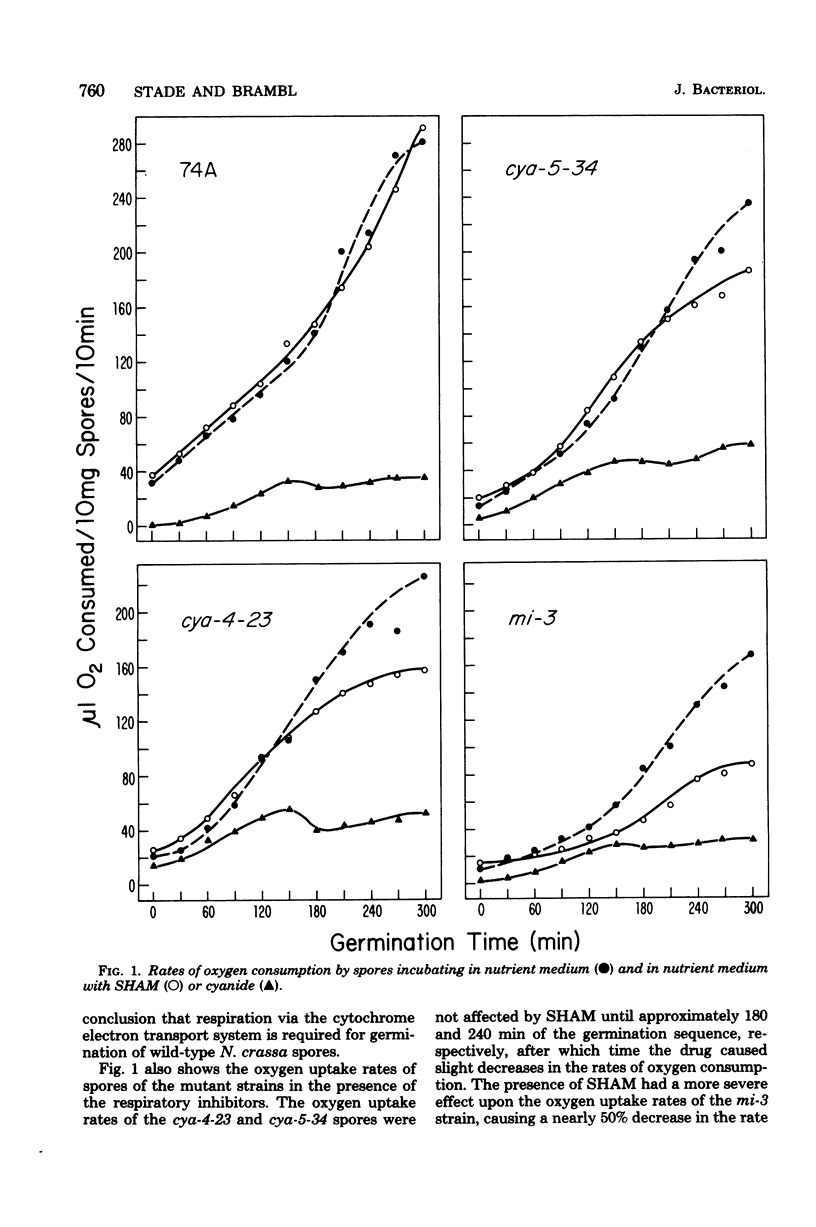
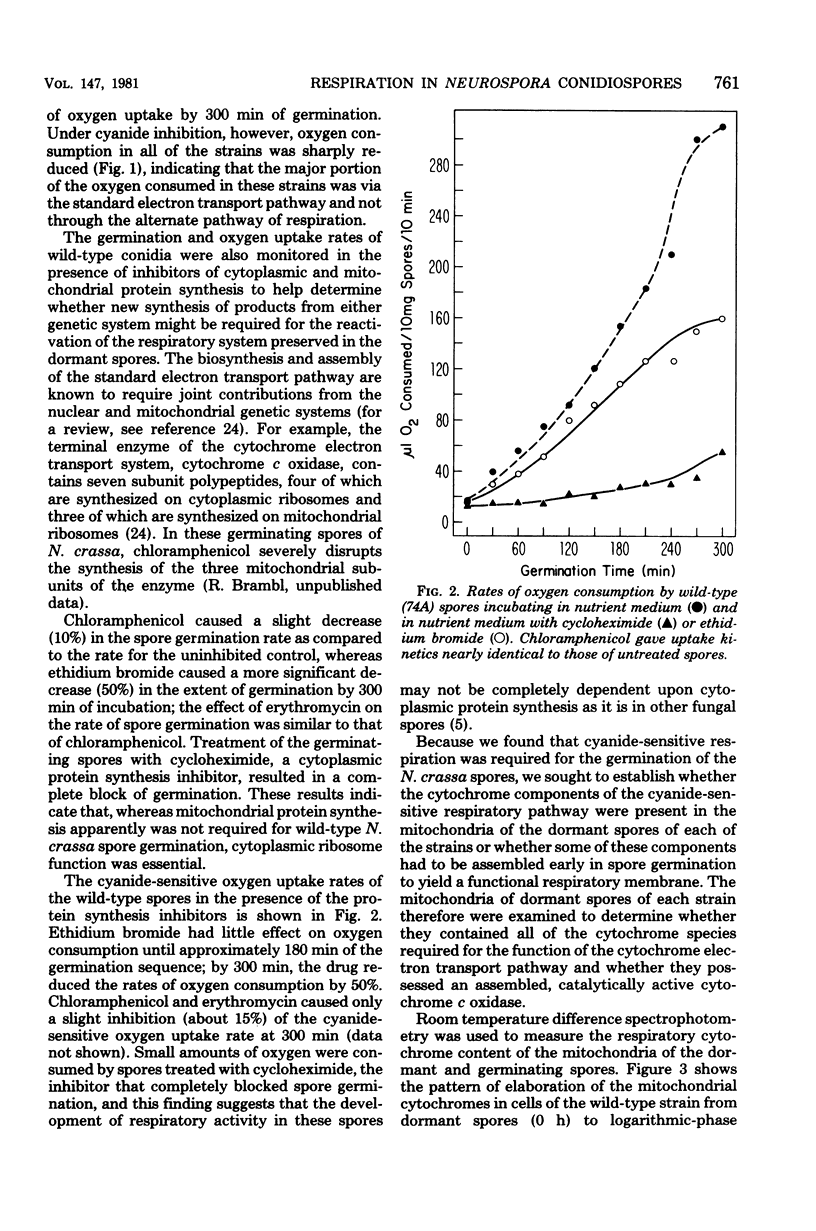
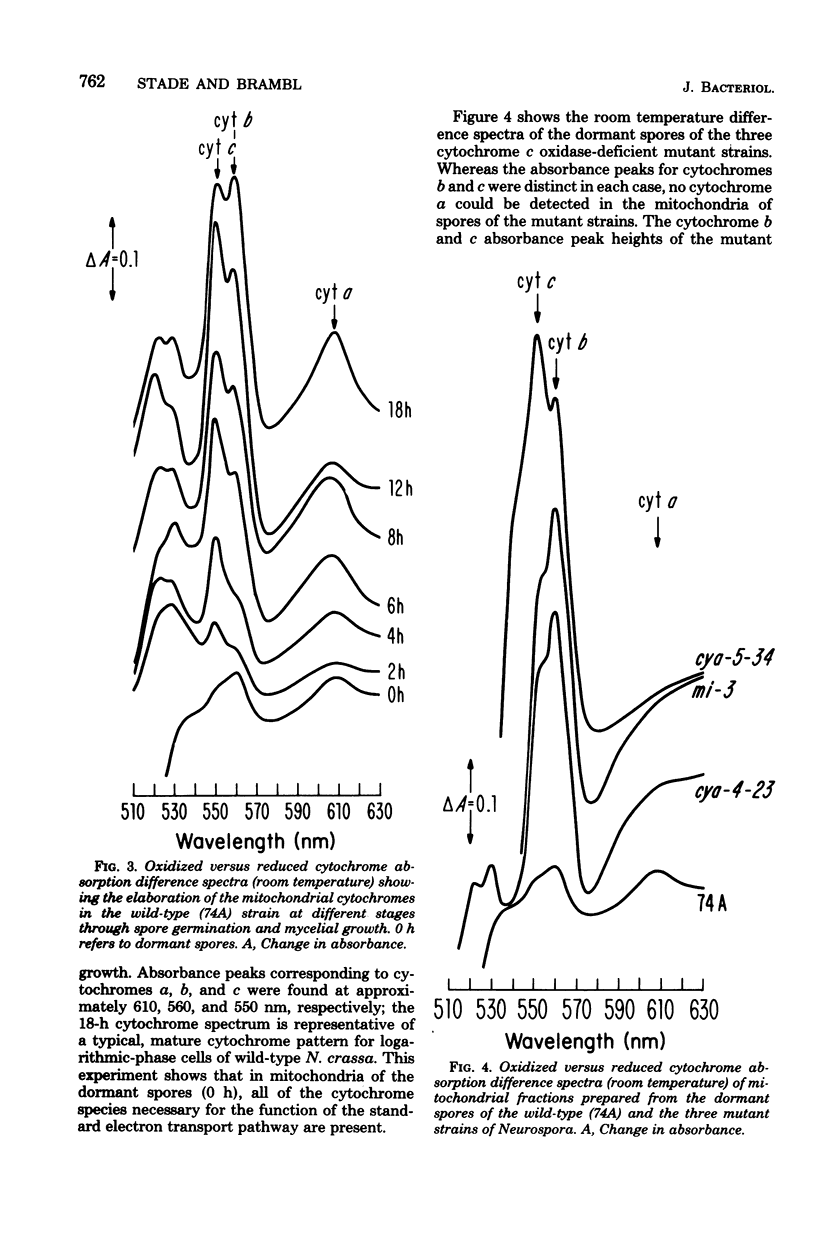
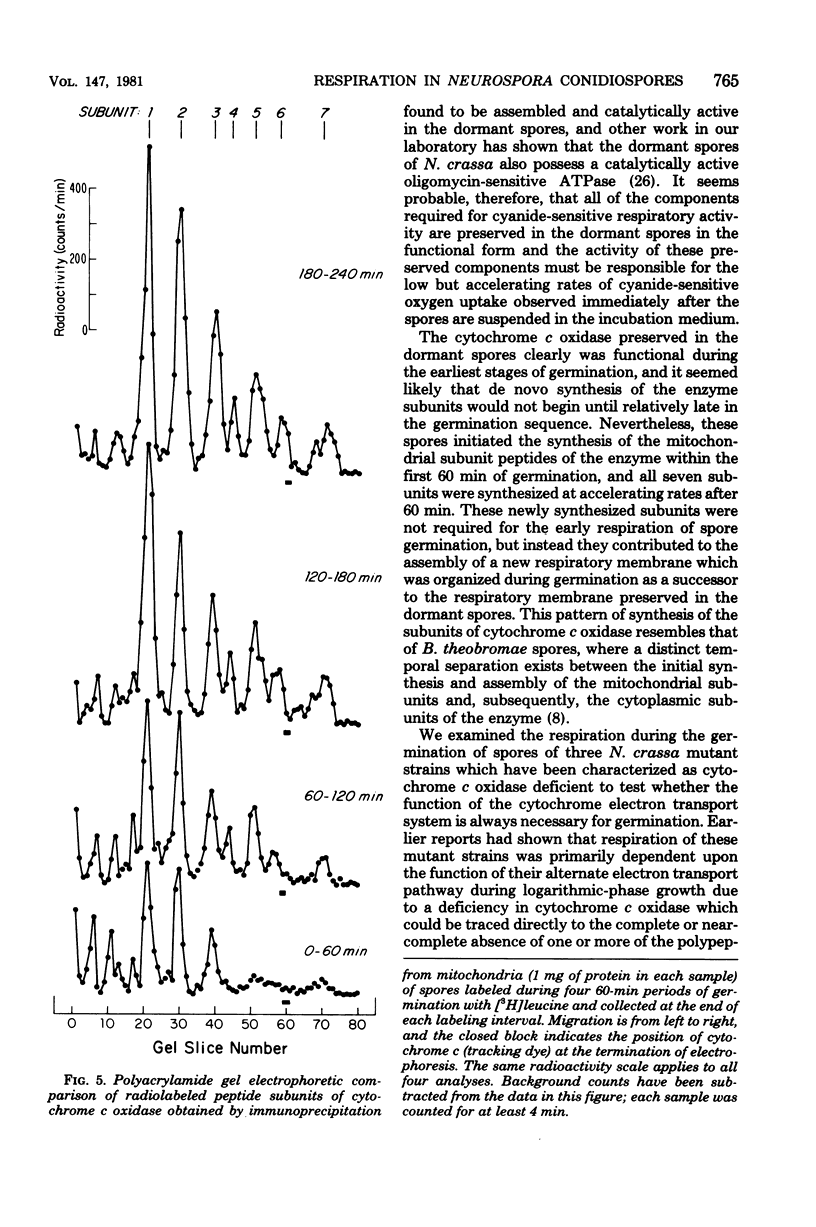
The germination of conidiospores of wild-type Neurospora crassa was found to be dependent upon the function of the cytochrome-mediated electron transport pathway. The cyanide-insensitive alternate oxidase did not contribute significantly to the respiration of these germinating spores. The dormant spores contained all of the cytochrome components and a catalytically active cytochrome c oxidase required for the activity of the standard respiratory pathway, and these preserved components were responsible for the accelerating rates of oxygen uptake which began immediately upon suspension of the spores in an incubation medium. Mitochondria of the dormant spores contained all of the subunit peptides of the functional cytochrome c oxidase; nevertheless, de novo synthesis of these subunits began at low rates in the first stages of germination. Reactivation of the respiratory system of germinating N. crassa spores seems not to be dependent initially upon the function of either the mitochondrial or cytoplasmic protein-synthesizing systems. The respiratory activity of spores of three mutant cytochrome c oxidase-deficient strains of N. crassa also was found to depend upon the function of the cytochrome electron transport pathway; the dormant and germinating spores of these strains contained a catalytically active cytochrome c oxidase. Cytochrome c oxidase may be present in the dormant and germinating spores of these strains as the result of a developmental-phase-specific synthesis of and requirement for the enzyme.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertrand H., Collins R. A. A regulatory system controlling the production of cytochrome aa3 in Neurospora crassa. Mol Gen Genet. 1978 Oct 25;166(1):1–13. doi: 10.1007/BF00379723. [DOI] [PubMed] [Google Scholar]
- Bertrand H., Nargang F. E., Collins R. A., Zagozeski C. A. Nuclear cytochrome-deficient mutants of Neurospora crassa: isolation, characterization, and genetic mapping. Mol Gen Genet. 1977 Jun 24;153(3):247–257. doi: 10.1007/BF00431590. [DOI] [PubMed] [Google Scholar]
- Bertrand H., Werner S. Cytochrome c oxidase subunits in nuclear and extranuclear cytochrome-aa3-deficient mutants of Neurospora crassa. Eur J Biochem. 1979 Jul;98(1):9–18. doi: 10.1111/j.1432-1033.1979.tb13154.x. [DOI] [PubMed] [Google Scholar]
- Bertrand H., Werner S. Deficiency of subunit two of cytochrome oxidase in the mi-3 cytoplasmic mutant of Neurospora crassa. Eur J Biochem. 1977 Oct 3;79(2):599–606. doi: 10.1111/j.1432-1033.1977.tb11844.x. [DOI] [PubMed] [Google Scholar]
- Brambl R. Characteristics of developing mitochondrial genetic and respiratory functions in germinating fungal spores. Biochim Biophys Acta. 1975 Aug 11;396(2):175–186. doi: 10.1016/0005-2728(75)90032-8. [DOI] [PubMed] [Google Scholar]
- Brambl R., Josephson M. Mitochondrial biogenesis during fungal spore germination: respiratory cytochromes of dormant and germinating spores of Botryodiplodia. J Bacteriol. 1977 Jan;129(1):291–297. doi: 10.1128/jb.129.1.291-297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambl R. Mitochondrial biogenesis during fungal spore germination. Biosynthesis and assembly of cytochrome c oxidase in Botryodiplodia theobromae. J Biol Chem. 1980 Aug 25;255(16):7673–7680. [PubMed] [Google Scholar]
- Brambl R. Mitochondrial biogenesis during fungal spore germination. Development of cytochrome c oxidase activity. Arch Biochem Biophys. 1977 Jul;182(1):273–281. doi: 10.1016/0003-9861(77)90308-3. [DOI] [PubMed] [Google Scholar]
- Brambl R. Presence of polyribosomes in condiospores of Botryodiplodia theobromae harvested with nonaqueous solvents. J Bacteriol. 1975 Jun;122(3):1394–1395. doi: 10.1128/jb.122.3.1394-1395.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONRAD H., SMITH L. A study of the kinetics of the oxidation of cytochrome c by cytochrome c oxidase. Arch Biochem Biophys. 1956 Aug;63(2):403–413. doi: 10.1016/0003-9861(56)90055-8. [DOI] [PubMed] [Google Scholar]
- Colvin H. J., Sauer B. L., Munkres K. D. Respiration of wild type and extrachromosomal mutants of Neurospora crassa. J Bacteriol. 1973 Dec;116(3):1314–1321. doi: 10.1128/jb.116.3.1314-1321.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley E. S., Greenawalt J. W. Biogenesis of mitochondrial membranes in Neurospora crassa. Mitochondrial protein synthesis during conidial germination. Eur J Biochem. 1975 Jun;54(2):585–601. doi: 10.1111/j.1432-1033.1975.tb04171.x. [DOI] [PubMed] [Google Scholar]
- Josephson M., Brambl R. Mitochondrial biogenesis during fungal spore germination. Purification, properties and biosynthesis of cytochrome c oxidase from Botryodiplodia theobromae. Biochim Biophys Acta. 1980;606(1):125–137. doi: 10.1016/0005-2787(80)90104-5. [DOI] [PubMed] [Google Scholar]
- Juretic D. Cyanide-resistant respiration of a Neurospora crassa membrane mutant. J Bacteriol. 1976 Apr;126(1):542–543. doi: 10.1128/jb.126.1.542-543.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mitchell M. B., Mitchell H. K., Tissieres A. Mendelian and Non-Mendelian Factors Affecting the Cytochrome System in Neurospora Crassa. Proc Natl Acad Sci U S A. 1953 Jul;39(7):606–613. doi: 10.1073/pnas.39.7.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargang F. E., Bertrand H., Werner S. A nuclear mutant of Neurospora crassa lacking subunit 1 of cytochrome c oxidase. J Biol Chem. 1978 Sep 25;253(18):6364–6369. [PubMed] [Google Scholar]
- Schmit J. C., Brody S. Biochemical genetics of Neurospora crassa conidial germination. Bacteriol Rev. 1976 Mar;40(1):1–41. doi: 10.1128/br.40.1.1-41.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayman C. W., Rees D. C., Orchard P. P., Slayman C. L. Generation of adenosine triphosphate in cytochrome-deficient mutants of Neurospora. J Biol Chem. 1975 Jan 25;250(2):396–408. [PubMed] [Google Scholar]
- Wenzler H., Brambl R. In vitro translation of polyadenylate-containing RNAs from dormant and germinating spores of the fungus Botryodiplodia theobromae. J Bacteriol. 1978 Jul;135(1):1–9. doi: 10.1128/jb.135.1.1-9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzler H., Brambl R. Mitochondrial biogenesis during fungal spore germination. Catalytic activity, composition, and subunit biosynthesis of oligomycin-sensitive ATPase in Botryodiplodia. J Biol Chem. 1981 Jul 25;256(14):7166–7172. [PubMed] [Google Scholar]
- von Jagow G., Weiss H., Klingenberg M. Comparison of the respiratory chain of Neurospora crassa wild type and the mi-mutants mi-1 and mi-3. Eur J Biochem. 1973 Feb 15;33(1):140–157. doi: 10.1111/j.1432-1033.1973.tb02665.x. [DOI] [PubMed] [Google Scholar]


