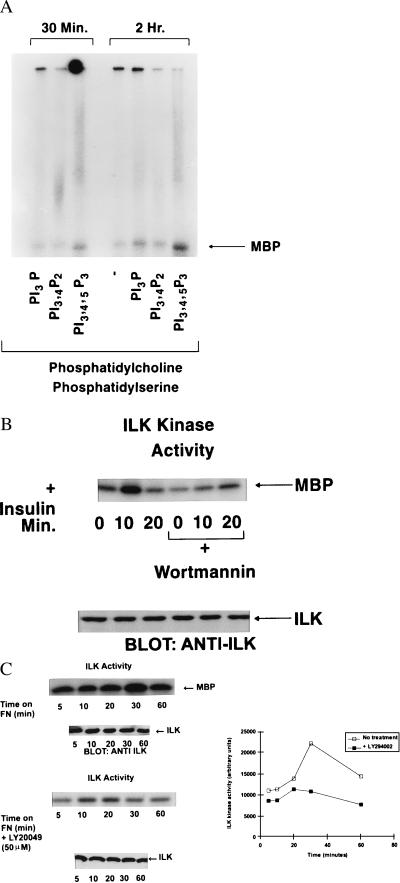
Figure 2.
(A) PtdIns(3, 4, 5)P3 specifically stimulates the activity of recombinant wild-type ILK in vitro. The indicated phospholipids were prepared as lipid vesicles as described in Materials and Methods and incubated with ILK. ILK activity was then determined by using γ32P-ATP and MBP as an exogenous substrate. Phosphorylated MBP was detected by SDS/PAGE and autoradiography. (B) Stimulation of ILK activity by insulin. IEC-18 cells were serum-starved overnight and then exposed to insulin (100 nM) for the indicated time periods. ILK was immunoprecipitated from cell extracts and ILK activity determined by using MBP as described in Materials and Methods. Pi(3)K activity was inhibited by preincubating the cells with Wortmannin (200 nM) for 20 min. Bottom shows equivalent amount of ILK in each extract as determined by Western blotting with an anti-ILK antibody. (C) Stimulation of ILK activity by cell adhesion on fibronectin. IEC-18 cells were serum-starved overnight, resuspended in serum-free medium, and allowed to adhere to fibronectin- (10 μg/ml) coated nontissue culture plastic plates for the indicated time periods. Nonadherent cells were removed, and adherent cells were lysed. ILK kinase activity was determined as described for B. Pi(3)K activity was inhibited by preincubating the cells with Ly294002 (50 μM) for 20 min. Bottom shows equivalent levels of ILK in each extract as determined by Western blotting with anti-ILK antibody. Quantification of the extent of phosphorylation was determined by phosphorimage analysis. Data shown in A, B, and C are representative of three independent experiments.