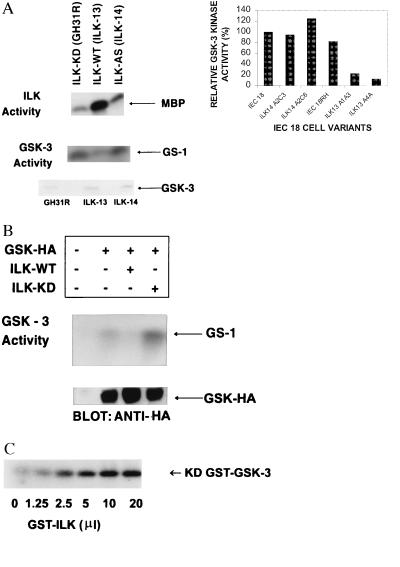
Figure 4.
ILK inhibits GSK-3 activity. (A) ILK and GSK-3 activities were measured in cell clones stably overexpressing wild-type ILK (ILK-13), kinase-deficient (KD) ILK (ILK-KD), or antisense ILK (ILK-14) (16–19) as described in Materials and Methods. Overexpression of wild-type ILK results in high ILK activity and drastic inhibition of GSK-3 activity. The level of expression of GSK-3 protein is similar in cell clones, as determined by Western blot analysis by using anti-GSK-3 antibody. (Inset) Quantification of GSK-3 activity in different IEC-18 clones. Data shown are the means of two separate experiments. (B) Transient transfection of ILK and HA-tagged GSK-3 in HEK-293 cells. Coexpression of wild-type ILK results in inhibition of GSK-3 activity whereas coexpression of kinase-deficient ILK (ILK-KD) results in enhanced GSK-3 activity relative to cells not transfected with ILK. Transfection efficiency was determined by monitoring GSK–HA expression by Western blot analysis by using anti-HA antibody. (C) ILK can phosphorylate GSK-3 in vitro. Recombinant kinase-deficient GST–GSK-3 coupled to glutathione-Sepharose beads was incubated with increasing concentrations of GST-ILK (7.5 ng/μl) in the presence of 32P-orthophosphate. After 2-hr incubation at 30°C, phosphorylated GSK was detected by SDS/PAGE and autoradiography. ILK did not phosphorylate GST.