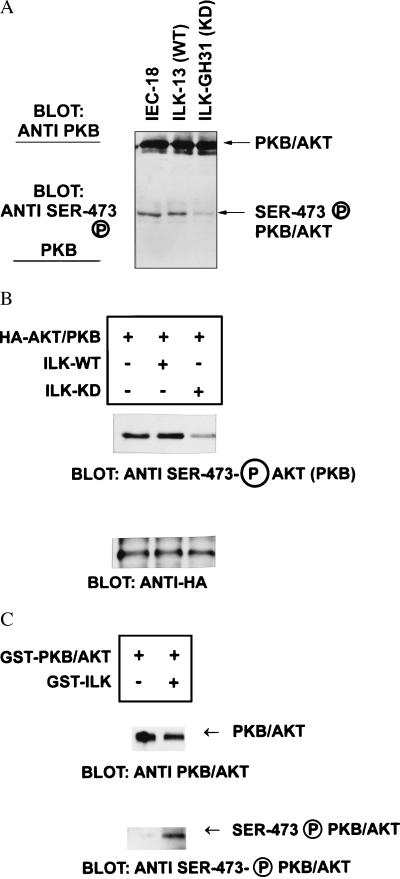
Figure 5.
Kinase-deficient ILK inhibits phosphorylation of PKB/AKT on serine-473, and wild-type ILK directly phosphorylates PKB/AKT on serine-473 in vitro. (A) The status of phosphorylation of PKB/AKT on serine-473 was determined in IEC-18 cells and in wild-type ILK overexpressing (ILK-13) cells or IEC-18 cells expressing kinase-deficient ILK (ILK-KD, GH31R) (18, 19). Cells growing in serum (5 μg/ml) containing tissue culture medium were lysed and extracts were analyzed by Western blotting by using either anti-PKB/AKT antibody or serine-473 phospho-specific antibody. PKB/AKT is expressed at equivalent levels in all cell lines but the extent of serine-473 phosphorylated PKB/AKT is dramatically inhibited in cells expressing kinase-deficient ILK. (B) Transient transfection of kinase-deficient ILK with HA-tagged PKB/AKT inhibits serine-473 phosphorylation of PKB/AKT. Cotransfection of PKB/AKT with wild-type ILK results in enhanced phosphorylation on serine-473, whereas cotransfection with kinase-deficient ILK results in inhibition of serine-473 phosphorylation of PKB/AKT. Transfection efficiency was monitored by analyzing PKB/AKT-HA expression by using Western blot analysis with anti-HA antibody. (C) Direct phosphorylation of PKB/AKT on serine-473 by ILK in vitro. Recombinant GST-PKB/AKT coupled to glutathione-Sepharose beads was incubated with or without recombinant GST-ILK (75 ng) in kinase reaction buffer. After 2 hr at 30°C, the GST-PKB/AKT beads were precipitated and analyzed by Western blot analysis. The amount of PKB/AKT detected is equivalent in the presence or absence of ILK, whereas serine-473 phosphorylated PKB/AKT is detectable only in the presence of ILK.