Abstract
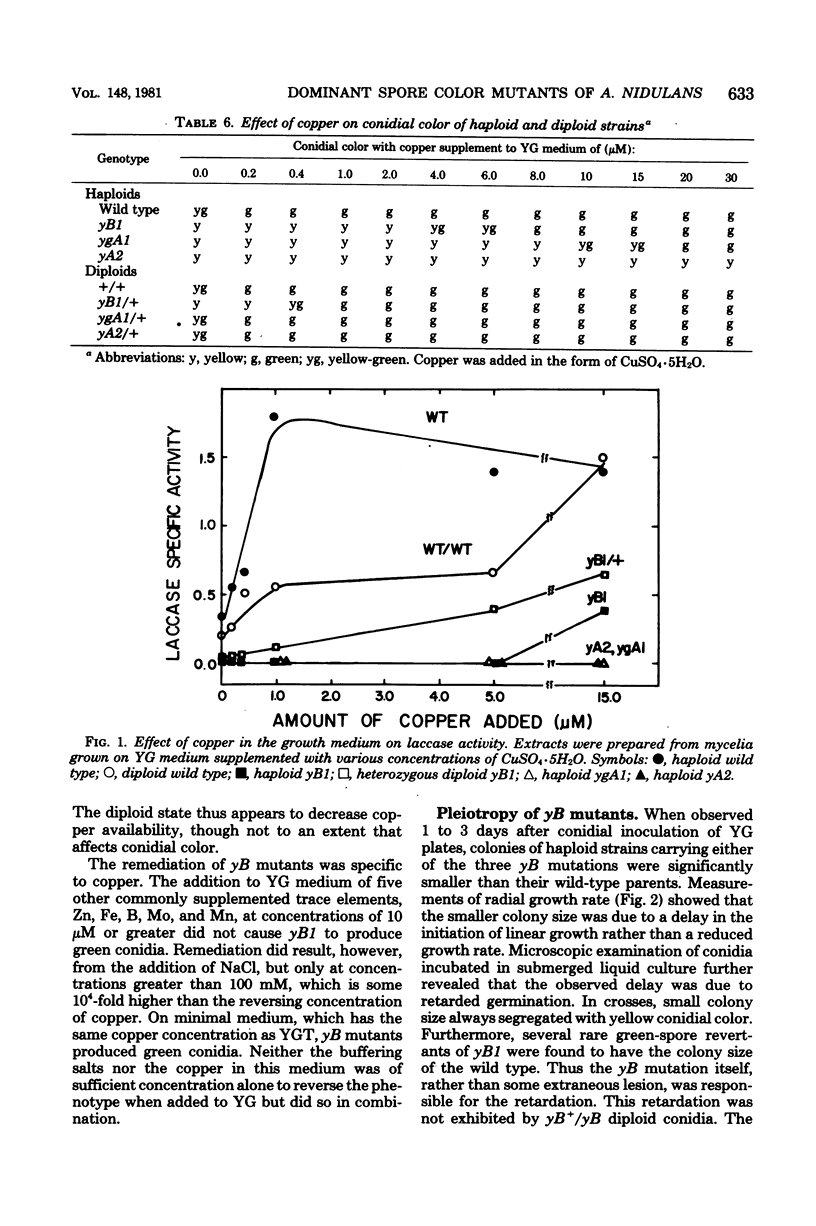
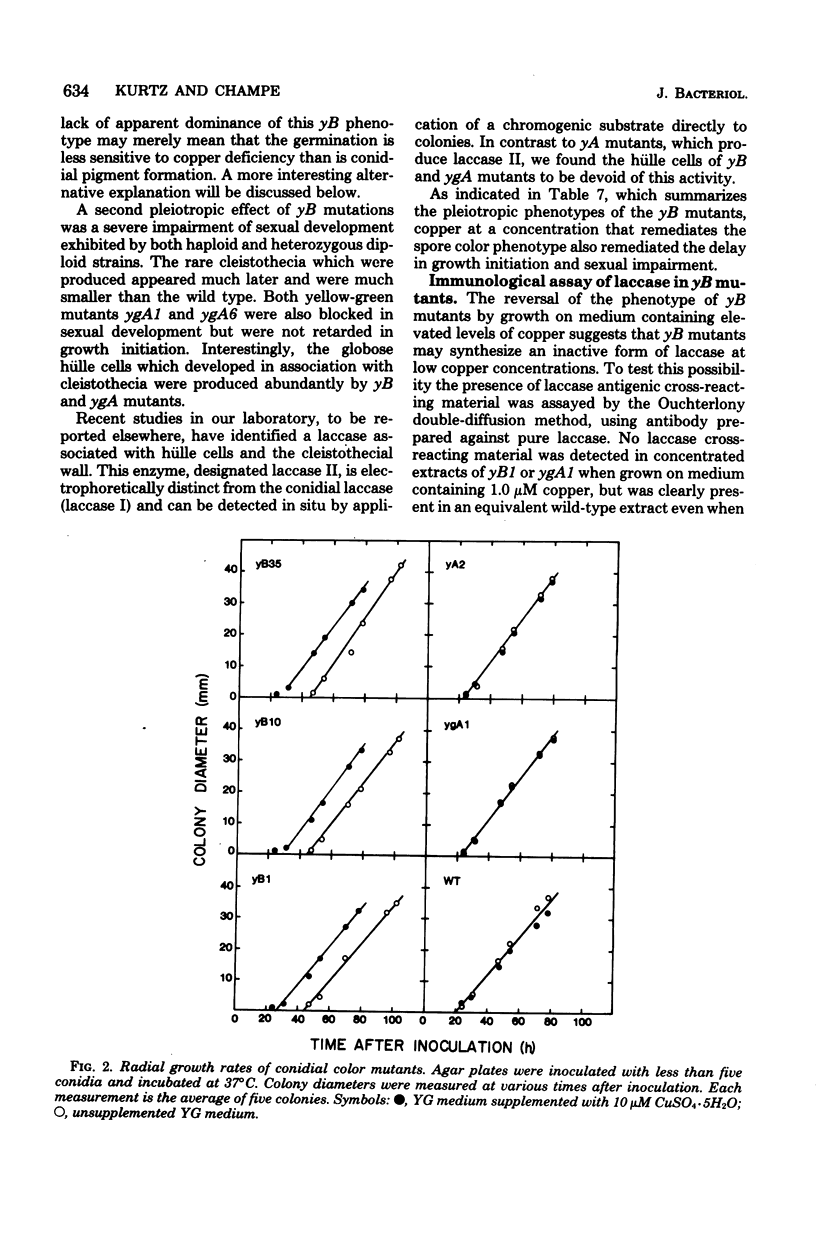
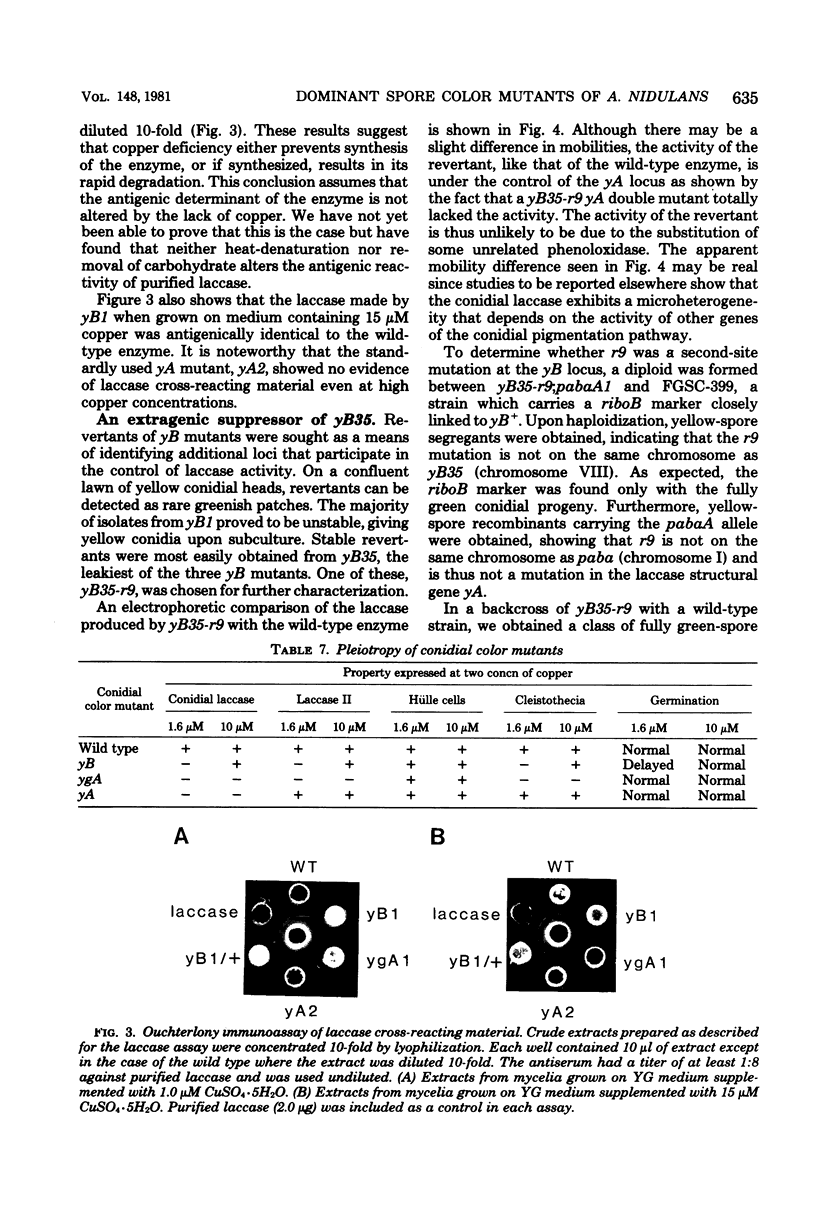
The ascomycete Aspergillus nidulans produces green conidia (asexual spores). Recessive mutants which produce yellow conidia have been previously isolated from haploid strains and have been shown to be deficient in laccase (diphenol oxidase), an enzyme that requires copper for activity. Using a diploid parent strain, we isolated dominant yellow conidial mutants which, in the haploid state, produced even less laccase activity than a recessive mutant. Three isolates of such mutants behaved similarly and define a single complementation group (yB) on chromosome VIII distinct from the yA locus on chromosome I defined by recessive mutants. Unlike yA mutants, whose only discernable phenotype is their conidial color, yB mutants are pleiotropic: conidial germination was delayed relative to the wild type, and sexual development was blocked at an early stage. The three phenotypes of yB mutants were expressed on yeast extract-glucose medium containing 1.6 microM of added copper. When copper was added to above 5 microM, all three phenotypes were remediated, and near wild-type levels of laccase were produced. We conclude that yB mutants have a reduced availability of copper. The dominance of yB mutants could result, for example, from an alteration in transport or storage of copper. Using an immunological assay, we detected no laccase antigenic cross-reacting material in yB mutants grown on medium of low copper content. We conclude that either the synthesis or the stability of laccase is copper dependent.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitt S., McCullough W., Roberts C. F. Analysis of acetate non-utilizing (acu) mutants in Aspergillus nidulans. J Gen Microbiol. 1976 Feb;92(2):263–282. doi: 10.1099/00221287-92-2-263. [DOI] [PubMed] [Google Scholar]
- Charlang G., Ng B., Horowitz N. H., Horowitz R. M. Cellular and extracellular siderophores of Aspergillus nidulans and Penicillium chrysogenum. Mol Cell Biol. 1981 Feb;1(2):94–100. doi: 10.1128/mcb.1.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutterbuck A. J. Absence of laccase from yellow-spored mutants of Aspergillus nidulans. J Gen Microbiol. 1972 May;70(3):423–435. doi: 10.1099/00221287-70-3-423. [DOI] [PubMed] [Google Scholar]
- Clutterbuck A. J. Gene symbols in Aspergillus nidulans. Genet Res. 1973 Jun;21(3):291–296. doi: 10.1017/s0016672300013483. [DOI] [PubMed] [Google Scholar]
- Cousins R. J. Synthesis and degradation of liver metallothionein. Experientia Suppl. 1979;34:293–301. doi: 10.1007/978-3-0348-6493-0_22. [DOI] [PubMed] [Google Scholar]
- Cove D. J. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta. 1966 Jan 11;113(1):51–56. doi: 10.1016/s0926-6593(66)80120-0. [DOI] [PubMed] [Google Scholar]
- Dorn G. L. A revised map of the eight linkage groups of Aspergillus nidulans. Genetics. 1967 Aug;56(4):619–631. doi: 10.1093/genetics/56.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser K., Minuth W. The phenoloxidases of the ascomycete Podospora anserina. Communication 4. Genetic regulation of the formation of laccase. Genetics. 1970 Mar-Apr;64(3):441–458. [PMC free article] [PubMed] [Google Scholar]
- Esser K. Phenol oxidases and morphogenesis in Podospora anserina. Genetics. 1968 Oct;60(2):281–288. doi: 10.1093/genetics/60.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz N. H., Charlang G., Horn G., Williams N. P. Isolation and identification of the conidial germination factor of Neurospora crassa. J Bacteriol. 1976 Jul;127(1):135–140. doi: 10.1128/jb.127.1.135-140.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurn B. A., Chantler S. M. Production of reagent antibodies. Methods Enzymol. 1980;70(A):104–142. doi: 10.1016/s0076-6879(80)70044-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law D. J., Timberlake W. E. Developmental regulation of laccase levels in Aspergillus nidulans. J Bacteriol. 1980 Nov;144(2):509–517. doi: 10.1128/jb.144.2.509-517.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard T. J. Phenoloxidase activity and fruiting body formation Schizophyllum commune. J Bacteriol. 1971 Apr;106(1):162–167. doi: 10.1128/jb.106.1.162-167.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard T. J., Phillips L. E. Study of phenoloxidase activity during the reproductive cycle in Schizophyllum commune. J Bacteriol. 1973 Apr;114(1):7–10. doi: 10.1128/jb.114.1.7-10.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch K. Copper metallothionein, a copper-binding protein from Neurospora crassa. Nature. 1980 Mar 27;284(5754):368–370. doi: 10.1038/284368a0. [DOI] [PubMed] [Google Scholar]
- Oudin J. Immunochemical analysis by antigen-antibody precipitation in gels. Methods Enzymol. 1980;70(A):166–198. doi: 10.1016/s0076-6879(80)70048-4. [DOI] [PubMed] [Google Scholar]
- PONTECORVO G., ROPER J. A., HEMMONS L. M., MACDONALD K. D., BUFTON A. W. J. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]